��Ŀ����
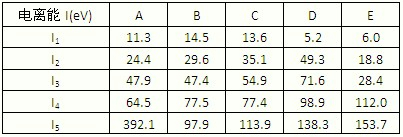
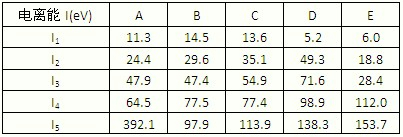
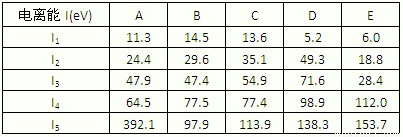
����ͼ1���±�Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ�

�û�ѧ����ش��������⣺
��1��д��Ԫ��f�Ļ�̬ԭ�Ӻ�������Ų�ʽ
��2����c6a6�����У�Ԫ��cΪ
��3��ci2���ӵĵ���ʽΪ
 ��ci2��ce2�Ƚϣ��е�ϸߵ���
��ci2��ce2�Ƚϣ��е�ϸߵ���
��4����һ�����ܣ�h
��5�����й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ��
A��jλ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ��
B��d�Ļ�̬ԭ���У�2p�ܼ�Ϊ�����������p��Ԫ��
C�����������Ų�ʽΪ4s1��һ�����ڢ�A��
D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B��
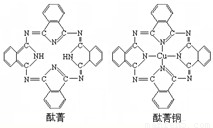
��6������ˮ���뵽j����������Һ�У��Ȳ�����ɫ������Ȼ��������ܽⲢ�õ�����ɫ��Һ��������ɫ��������
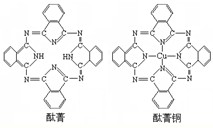
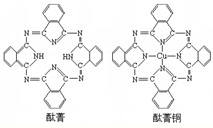
��7��j�Ľ�������ľ�����ͼ2��ʾ����һ��������jԭ�ӵĸ�����

�û�ѧ����ش��������⣺
��1��д��Ԫ��f�Ļ�̬ԭ�Ӻ�������Ų�ʽ
1s22s22p5
1s22s22p5
����2����c6a6�����У�Ԫ��cΪ
sp2
sp2
�ӻ����÷������Ǽ���
�Ǽ���
���ӣ�����ԡ��Ǽ��ԡ�������3��ci2���ӵĵ���ʽΪ


CS2
CS2
��д����ʽ������4����һ�����ܣ�h
��
��
i���縺�ԣ�g��
��
b�����������������=��������5�����й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ��
AB
AB
��A��jλ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ��
B��d�Ļ�̬ԭ���У�2p�ܼ�Ϊ�����������p��Ԫ��
C�����������Ų�ʽΪ4s1��һ�����ڢ�A��
D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B��
��6������ˮ���뵽j����������Һ�У��Ȳ�����ɫ������Ȼ��������ܽⲢ�õ�����ɫ��Һ��������ɫ��������
[Cu��NH3��4]2+
[Cu��NH3��4]2+
��д����ɫ�����ܽ��ڰ�ˮ�е����ӷ���ʽCu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O
Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O
����7��j�Ľ�������ľ�����ͼ2��ʾ����һ��������jԭ�ӵĸ�����
4
4
��������������Ԫ�������ڱ��е�λ�ÿ�֪��aΪHԪ�ء�bΪBeԪ�ء�cΪCԪ�ء�dΪNԪ�ء�eΪOԪ�ء�fΪFԪ�ء�gΪNaԪ�ء�hΪPԪ�ء�iΪSԪ�ء�jΪCuԪ�أ�
��1��fΪFԪ�أ�ԭ�Ӻ��������ĿΪ9�����ݺ�������Ų�������д��
��2��c6a6������C6H6��Cԭ�ӳ�3���Ҽ��������д���1����м�������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
��3��ci2����CS2���������̼�Ľṹ���ƣ����߽ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ�
��4��PԪ�ص�3p�ܼ�Ϊ�����ȶ�״̬���������ͣ���һ�����ܽϸߣ�������Խǿ�縺��ԽС��
��5��A��j��CuԪ��λ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�أ�
B��Nԭ�ӵĻ�̬ԭ����2p�ܼ�Ϊ�����������p��Ԫ�أ�
C�����������Ų�ʽΪ4s1�����ܴ��ڢ�A�塢��B�塢��B�壻
D�����������Ų�ʽΪns2np1����Ԫ�ش��ڢ�A�壻
��6��������ͭ�����ڰ�ˮ�γ��İ���ͭ�����ӣ�
��7�����ݾ�̯�����㾧����ԭ����Ŀ��
��1��fΪFԪ�أ�ԭ�Ӻ��������ĿΪ9�����ݺ�������Ų�������д��
��2��c6a6������C6H6��Cԭ�ӳ�3���Ҽ��������д���1����м�������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
��3��ci2����CS2���������̼�Ľṹ���ƣ����߽ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ�
��4��PԪ�ص�3p�ܼ�Ϊ�����ȶ�״̬���������ͣ���һ�����ܽϸߣ�������Խǿ�縺��ԽС��
��5��A��j��CuԪ��λ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�أ�
B��Nԭ�ӵĻ�̬ԭ����2p�ܼ�Ϊ�����������p��Ԫ�أ�
C�����������Ų�ʽΪ4s1�����ܴ��ڢ�A�塢��B�塢��B�壻
D�����������Ų�ʽΪns2np1����Ԫ�ش��ڢ�A�壻
��6��������ͭ�����ڰ�ˮ�γ��İ���ͭ�����ӣ�
��7�����ݾ�̯�����㾧����ԭ����Ŀ��
����⣺����Ԫ�������ڱ��е�λ�ÿ�֪��aΪHԪ�ء�bΪBeԪ�ء�cΪCԪ�ء�dΪNԪ�ء�eΪOԪ�ء�fΪFԪ�ء�gΪNaԪ�ء�hΪPԪ�ء�iΪSԪ�ء�jΪCuԪ�أ�
��1��fΪFԪ�أ�ԭ�Ӻ��������ĿΪ9����������Ų�ʽΪ1s22s22p5���ʴ�Ϊ��1s22s22p5��
��2��c6a6������C6H6��Cԭ�ӳ�3���Ҽ�����ȡsp2�ӻ�������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
�ʴ�Ϊ��sp2���Ǽ��ԣ�
��3��ci2����CS2���������̼�Ľṹ���ƣ�����ʽΪ ��CS2��CO2�ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ���CS2�е�ϸߣ�
��CS2��CO2�ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ���CS2�е�ϸߣ�
�ʴ�Ϊ�� ��CS2��
��CS2��
��4��PԪ�ص�3p�ܼ�Ϊ�����ȶ�״̬���������ͣ��ʵ�һ������P��S��������Խǿ���縺��ԽС���ʵ縺��Na��Be��
�ʴ�Ϊ����������
��5��A��j��CuԪ��λ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�أ���A��ȷ��
B��Nԭ�ӵĻ�̬ԭ����2p�ܼ�Ϊ�����������p��Ԫ�أ���B��ȷ��
C�����������Ų�ʽΪ4s1�����ܴ��ڢ�A�塢��B�塢��B�壬��B����
D�����������Ų�ʽΪns2np1����Ԫ�ش��ڢ�A�壬��D����
�ʴ�Ϊ��AB��
��6��������ͭ�����ڰ�ˮ�γ�[Cu��NH3��4]2+�����ӷ���ʽΪ��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
�ʴ�Ϊ��[Cu��NH3��4]2+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
��7����Cu������֪�������к���Cuԭ����ĿΪ6��
+8��
=4��
�ʴ�Ϊ��4��
��1��fΪFԪ�أ�ԭ�Ӻ��������ĿΪ9����������Ų�ʽΪ1s22s22p5���ʴ�Ϊ��1s22s22p5��
��2��c6a6������C6H6��Cԭ�ӳ�3���Ҽ�����ȡsp2�ӻ�������Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�
�ʴ�Ϊ��sp2���Ǽ��ԣ�
��3��ci2����CS2���������̼�Ľṹ���ƣ�����ʽΪ
 ��CS2��CO2�ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ���CS2�е�ϸߣ�
��CS2��CO2�ṹ���ƣ��������γɷ��Ӿ��壬��Է�������Խ�е�Խ�ߣ���CS2�е�ϸߣ��ʴ�Ϊ��
 ��CS2��
��CS2����4��PԪ�ص�3p�ܼ�Ϊ�����ȶ�״̬���������ͣ��ʵ�һ������P��S��������Խǿ���縺��ԽС���ʵ縺��Na��Be��
�ʴ�Ϊ����������
��5��A��j��CuԪ��λ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�أ���A��ȷ��
B��Nԭ�ӵĻ�̬ԭ����2p�ܼ�Ϊ�����������p��Ԫ�أ���B��ȷ��
C�����������Ų�ʽΪ4s1�����ܴ��ڢ�A�塢��B�塢��B�壬��B����
D�����������Ų�ʽΪns2np1����Ԫ�ش��ڢ�A�壬��D����
�ʴ�Ϊ��AB��
��6��������ͭ�����ڰ�ˮ�γ�[Cu��NH3��4]2+�����ӷ���ʽΪ��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
�ʴ�Ϊ��[Cu��NH3��4]2+��Cu��OH��2+4NH3?H2O=[Cu��NH3��4]2++2OH-+4H2O��
��7����Cu������֪�������к���Cuԭ����ĿΪ6��
| 1 |
| 2 |
| 1 |
| 8 |
�ʴ�Ϊ��4��
�����������Ƕ����ʽṹ�������ۺϿ��飬֪ʶ�㿼��ȫ�棬�ѶȲ�������֪ʶ������У�ע�����֪ʶ��ȫ�����գ�

��ϰ��ϵ�д�
 53������ϵ�д�
53������ϵ�д�
�����Ŀ