��Ŀ����
I�������£���amolN2��bmolH2�Ļ������ͨ��һ�������ݻ����ܱ������У��������·�Ӧ��N2��g��+3H2��g��?2NH3��g��
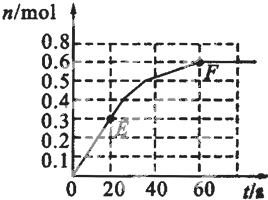
��1������Ӧ���е�ijʱ��tʱ��nt��N2��=13mol��nt��NH3��=6mol������a=______��
��2����Ӧ�ﵽƽ��ʱ�������������Ϊ716.8L������£�������NH3�ĺ��������������Ϊ25%������ƽ��ʱNH3�����ʵ���______��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȣ���ͬ����n��ʼ����n��ƽ��=______��
��4��ԭ��������У�a��b=______��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a��N2����a��H2��=______��
��6��ƽ���������У�n��N2����n��H2����n��NH3��=______��
II���������������ܱ������г���2molN2��6molH2��һ�������·�����Ӧ��
N2��g��+3H2��g��?2NH3��g����ƽ��ʱ�������7mol����a��b��c�ֱ����N2��H2��NH3��ʼ��������ʵ�����ά���¶Ȳ��䣬ʹ�ﵽƽ��ʱ���ɷֵİٷֺ������䣮��
��1����a=0��b=0����c=______��
��2����a=0.7��b=2.1����
��c=______��
����ʱ��Ӧ��______���У���Ϊ��______��
����Ҫά�ַ�Ӧ��ʼ��÷�Ӧ������У�c�ķ�Χ��______��
��3����ʹ��ʼ��Ӧά��������෴�ķ�����У���b�ķ�Χ��______��
��1������Ӧ���е�ijʱ��tʱ��nt��N2��=13mol��nt��NH3��=6mol������a=______��
��2����Ӧ�ﵽƽ��ʱ�������������Ϊ716.8L������£�������NH3�ĺ��������������Ϊ25%������ƽ��ʱNH3�����ʵ���______��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȣ���ͬ����n��ʼ����n��ƽ��=______��
��4��ԭ��������У�a��b=______��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ�a��N2����a��H2��=______��
��6��ƽ���������У�n��N2����n��H2����n��NH3��=______��
II���������������ܱ������г���2molN2��6molH2��һ�������·�����Ӧ��
N2��g��+3H2��g��?2NH3��g����ƽ��ʱ�������7mol����a��b��c�ֱ����N2��H2��NH3��ʼ��������ʵ�����ά���¶Ȳ��䣬ʹ�ﵽƽ��ʱ���ɷֵİٷֺ������䣮��
��1����a=0��b=0����c=______��
��2����a=0.7��b=2.1����
��c=______��
����ʱ��Ӧ��______���У���Ϊ��______��
����Ҫά�ַ�Ӧ��ʼ��÷�Ӧ������У�c�ķ�Χ��______��
��3����ʹ��ʼ��Ӧά��������෴�ķ�����У���b�ķ�Χ��______��
��1���ɷ�Ӧ�Ļ�ѧ����ʽ��֪����Ӧ����N2������NH3�����ʵ���֮��Ϊ1��2����ת����N2�����ʵ���Ϊxmol��
��x��6=1��2����֮x=3������a=13+3=16mol���ʴ�Ϊ��16��
��2����n=
=32mol��NH3�ĺ��������������Ϊ25%��
nƽ��NH3��=32mol��25%=8mol���ʴ�Ϊ��8mol��
��3��N2��g��+3H2��g�� 2NH3��g����
2NH3��g����
��ʼ16 b 0
ת��412 8
ƽ��12��32-12-8��8
n��ʼ����n��ƽ��=��16+24��������12+12+8��=40��32=5��4���ʴ�Ϊ��5��4��
��4������������֪��b=12+��32-12-8��=24mol��a=16mol������a��b=16mol��24mol=2��3���ʴ�Ϊ��2��3��
��5���ﵽƽ��ʱ��N2��H2��ת����֮��a��N2����a��H2��=
��0.5=1��2���ʴ�Ϊ��1��2��
��6��ƽ���������У�n��N2����n��H2����n��NH3��=12mol��12mol��8mol=3��3��2���ʴ�Ϊ��3��3��2��
II����1��N2��g��+3H2��g��?2NH3��g����
��ʼ 2 6 0
��ʼ0 0 4
���¶ȡ��������������£��ﵽ��ͬ��ƽ��״̬����c=4mol���ʴ�Ϊ��4mol��
��2���ٸ���N2��g��+3H2��g��?2NH3��g����
��ʼ0.7 2.1 c
ת��0.5c
��c����ת��Ϊa����0.5c+0.7=2�����c=2.6���ʴ�Ϊ��2.6��
�ڸ���N2��g��+3H2��g��?2NH3��g����
��ʼ2 6 0
ת��x 3x 2x
ƽ�� 2-x 6-3x 2x
2-x+6-3x+2x=7�����x=0.5��
��ƽ��ʱ���ʵ����ֱ�Ϊ1.5mol��4.5mol��1mol��
��a��b��c�ֱ�Ϊ0.7��2.1��2.6ʱ��Ũ���̣�ƽ�ⳣ����Q��K������ѧ��Ӧ�����ƶ���
�ʴ�Ϊ���淴Ӧ����2�֣���Ũ���̣�ƽ�ⳣ����
����ƽ��ʱcΪ1��c�����ֵΪ4����Ҫά�ַ�Ӧ��ʼ��÷�Ӧ������У�Ӧ����1��c��4���ʴ�Ϊ��1��c��4��
��3����ʹ��ʼ��Ӧά��������෴�ķ�����У�����Ӧ�������ƶ���ƽ��ʱb=4.5��b�����ֵΪ6��������4.5��b��6���ɣ�
�ʴ�Ϊ��4.5��b��6��
��x��6=1��2����֮x=3������a=13+3=16mol���ʴ�Ϊ��16��
��2����n=
| 716.8L |
| 22.4L/mol |
nƽ��NH3��=32mol��25%=8mol���ʴ�Ϊ��8mol��
��3��N2��g��+3H2��g��
 2NH3��g����
2NH3��g������ʼ16 b 0
ת��412 8
ƽ��12��32-12-8��8
n��ʼ����n��ƽ��=��16+24��������12+12+8��=40��32=5��4���ʴ�Ϊ��5��4��
��4������������֪��b=12+��32-12-8��=24mol��a=16mol������a��b=16mol��24mol=2��3���ʴ�Ϊ��2��3��
��5���ﵽƽ��ʱ��N2��H2��ת����֮��a��N2����a��H2��=
| 4 |
| 16 |
��6��ƽ���������У�n��N2����n��H2����n��NH3��=12mol��12mol��8mol=3��3��2���ʴ�Ϊ��3��3��2��
II����1��N2��g��+3H2��g��?2NH3��g����
��ʼ 2 6 0
��ʼ0 0 4
���¶ȡ��������������£��ﵽ��ͬ��ƽ��״̬����c=4mol���ʴ�Ϊ��4mol��
��2���ٸ���N2��g��+3H2��g��?2NH3��g����
��ʼ0.7 2.1 c
ת��0.5c
��c����ת��Ϊa����0.5c+0.7=2�����c=2.6���ʴ�Ϊ��2.6��
�ڸ���N2��g��+3H2��g��?2NH3��g����
��ʼ2 6 0
ת��x 3x 2x
ƽ�� 2-x 6-3x 2x
2-x+6-3x+2x=7�����x=0.5��
��ƽ��ʱ���ʵ����ֱ�Ϊ1.5mol��4.5mol��1mol��
��a��b��c�ֱ�Ϊ0.7��2.1��2.6ʱ��Ũ���̣�ƽ�ⳣ����Q��K������ѧ��Ӧ�����ƶ���
�ʴ�Ϊ���淴Ӧ����2�֣���Ũ���̣�ƽ�ⳣ����
����ƽ��ʱcΪ1��c�����ֵΪ4����Ҫά�ַ�Ӧ��ʼ��÷�Ӧ������У�Ӧ����1��c��4���ʴ�Ϊ��1��c��4��
��3����ʹ��ʼ��Ӧά��������෴�ķ�����У�����Ӧ�������ƶ���ƽ��ʱb=4.5��b�����ֵΪ6��������4.5��b��6���ɣ�
�ʴ�Ϊ��4.5��b��6��

��ϰ��ϵ�д�
�����Ŀ
 C��D����t1ʱ����ѹǿ�������淴Ӧ���ʱ仯ͼ����ͼ��ʾ�������й�A��B��C��D��״̬������ȷ���ǣ� ��
C��D����t1ʱ����ѹǿ�������淴Ӧ���ʱ仯ͼ����ͼ��ʾ�������й�A��B��C��D��״̬������ȷ���ǣ� ��