��Ŀ����
ij�¶��£���ӦH2��g��+CO2��g��?H2O��g��+CO��g����ƽ�ⳣ��K=
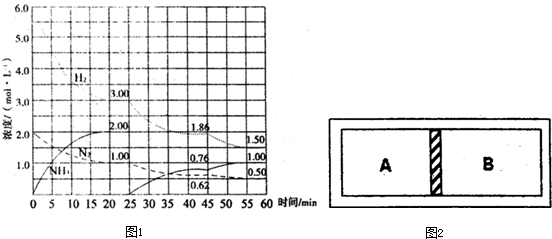
�����¶����������Ϊ10L�������ܱ������зֱ���뷴Ӧ���ʼ����������ͼ��ʾ��
�����жϲ���ȷ���ǣ�������
| 9 |
| 4 |
| ��ʼ�� | �� | �� | �� |
| H2��mol�� | 1 | 2 | 2 |
| CO2��mol�� | 1 | 1 | 2 |
| A����Ӧ��ʼʱ�����еķ�Ӧ�������������еķ�Ӧ������� |
| B��ƽ��ʱ���ͱ���CO2��ת������ȣ�����60% |
| C��ƽ��ʱ������H2��ת���ʴ���60% |
| D��ƽ��ʱ������c��H2����0.08mol?L-1 |
A���¶���ͬʱ�����ұ���Ӧ��Ũ����������Ӧ����Ҳ��������A����
B����������c��CO2��=c��H2��=
=0.1mol?L-1����H2ת����Ũ��Ϊxmol?L-1��
H2��g��+CO2��g��?H2O��g��+CO��g��
��ʼ��1mol?L-1�� 0.10.1 00
ת����1mol?L-1�� x x x x
ƽ�⣨1mol?L-1�� 0.1-x 0.1-x x x
��ѧƽ�ⳣ��k=
=
��x=0.06mol?L-1
CO2��ת����=H2��ת����
��100%=60%��
��ѧ��Ӧǰ���������ķ�Ӧ������ѹǿ����ѧƽ�ⲻ�ƶ������ʵ�ת���ʲ��䣬���ݼ���ó�����H2��ת���ʾ���60%�����Ա���H2��ת���ʾ���60%����B����
C���Һͼ���ȣ��൱���ڼĻ�������������������������������ת����С�ڼ��е�ת���ʣ���С��60%����C��ȷ��
D��ƽ��ʱ������c��H2��=��0.1-0.06��mol?L-1=0.04mol?L-1�����Ա���c��H2��=0.08mol?L-1����D����
��ѡC��
B����������c��CO2��=c��H2��=
| 1mol |
| 10L |
H2��g��+CO2��g��?H2O��g��+CO��g��
��ʼ��1mol?L-1�� 0.10.1 00
ת����1mol?L-1�� x x x x
ƽ�⣨1mol?L-1�� 0.1-x 0.1-x x x
��ѧƽ�ⳣ��k=
| x2 |
| (0.1-x)2 |
| 9 |
| 4 |
CO2��ת����=H2��ת����
| 0.06mol?L-1 |
| 0.1mol?L-1 |
��ѧ��Ӧǰ���������ķ�Ӧ������ѹǿ����ѧƽ�ⲻ�ƶ������ʵ�ת���ʲ��䣬���ݼ���ó�����H2��ת���ʾ���60%�����Ա���H2��ת���ʾ���60%����B����
C���Һͼ���ȣ��൱���ڼĻ�������������������������������ת����С�ڼ��е�ת���ʣ���С��60%����C��ȷ��
D��ƽ��ʱ������c��H2��=��0.1-0.06��mol?L-1=0.04mol?L-1�����Ա���c��H2��=0.08mol?L-1����D����
��ѡC��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 2C��g��������Ӧ�ﻯѧƽ��ʱ�������50%��Bת��ΪC����Ӧǰ��ƽ��ʱ�����ڵķ�����֮���ǣ� ��
2C��g��������Ӧ�ﻯѧƽ��ʱ�������50%��Bת��ΪC����Ӧǰ��ƽ��ʱ�����ڵķ�����֮���ǣ� �� 2Z(g)
2Z(g)