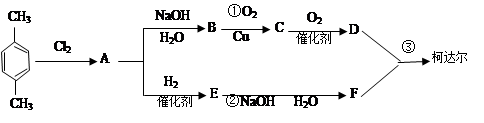��Ŀ����
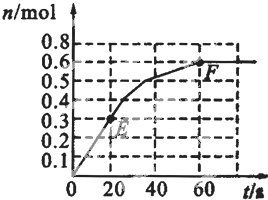
373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A��g��?2B��g������������B�����ʵ����仯��ͼ��ʾ
��1����֪373Kʱ60s�ﵽƽ�⣬��ǰ60s��A��ƽ����Ӧ����Ϊ______��
��2����373KʱB��ƽ��Ũ��ΪA��3����473Kʱ�������������䣩��B��ƽ��Ũ��ΪA��2����������ͼ�л���473KʱA�����ʵ�����ʱ��ı仯���ߣ�
��3������Ӧ��373K���У���1L�ܱ������м���1molB��0.2molHe���ﵽƽ��ʱB��ת����Ӧ______��
A������60%B������40%C��С��40%D������40%��60%֮��
��4����֪��������������֮����ߵ�б�ʱ�ʾ��ʱ����B��ƽ����Ӧ���ʣ�����ֱ��EF��б�ʱ�ʾ20s��60s��B��ƽ����Ӧ���ʣ����Բ�������������һ�������б�ʵ�����______��
��1����֪373Kʱ60s�ﵽƽ�⣬��ǰ60s��A��ƽ����Ӧ����Ϊ______��
��2����373KʱB��ƽ��Ũ��ΪA��3����473Kʱ�������������䣩��B��ƽ��Ũ��ΪA��2����������ͼ�л���473KʱA�����ʵ�����ʱ��ı仯���ߣ�
��3������Ӧ��373K���У���1L�ܱ������м���1molB��0.2molHe���ﵽƽ��ʱB��ת����Ӧ______��
A������60%B������40%C��С��40%D������40%��60%֮��
��4����֪��������������֮����ߵ�б�ʱ�ʾ��ʱ����B��ƽ����Ӧ���ʣ�����ֱ��EF��б�ʱ�ʾ20s��60s��B��ƽ����Ӧ���ʣ����Բ�������������һ�������б�ʵ�����______��

��1����ͼ��֪��ǰ60s��B�����ʵ����仯Ϊ0.6mol�����ݷ���ʽA��g��?2B��g����֪��n��A��=
��0.6mol=0.3mol������v��A��=
=0.005mol/��L?s����
�ʴ�Ϊ��0.005mol/��L?s����
��2����ͼ��֪��60s��ƽ��ʱB�����ʵ����仯Ϊ0.6mol�����ݷ���ʽA��g��?2B��g����֪��n��A��=0.3mol��B��ƽ��Ũ��ΪA��3����������䣬Ũ��֮�ȵ������ʵ���֮�ȣ��ʴ�ʱA�����ʵ���0.6mol��
=0.2mol������A����ʼ���ʵ���Ϊ0.3mol+0.2mol=0.5mol��
473Kʱ�������������䣩��B��ƽ��Ũ��ΪA��2����������䣬Ũ��֮�ȵ������ʵ���֮�ȣ���ƽ��ʱB�����ʵ���Ϊamol����A�����ʵ����仯Ϊ0.5amol���ʣ�0.5mol-0.5amol����2=amol�����a=0.5����ƽ��ʱA�����ʵ���Ϊ0.5��0.5mol=0.25mol���¶����߷�Ӧ���ʼӿ죬��473Kʱ��Ӧ����ƽ���ʱ��С��60s��ͼ��Ϊ��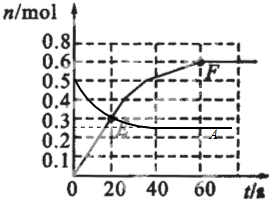
�ʴ�Ϊ�� ��
��
��3�����º���ϡ�����岻Ӱ��ƽ�⣬����1molB����ЧΪ����0.5molA����ԭƽ���Ч��ƽ��ʱB�����ʵ�����ͬΪ0.6mol���μӷ�ӦB�����ʵ���Ϊ1mol-0.6mol=0.4mol����B��ת����Ϊ
��100%=40%����ѡB��
��4������������һ�������б�ʱ�ʾ��ʱ�̵ļ�ʱ��Ӧ���ʣ��ʴ�Ϊ����ʾ��ʱ�̵ļ�ʱ��Ӧ���ʣ�
| 1 |
| 2 |
| ||
| 60s |
�ʴ�Ϊ��0.005mol/��L?s����
��2����ͼ��֪��60s��ƽ��ʱB�����ʵ����仯Ϊ0.6mol�����ݷ���ʽA��g��?2B��g����֪��n��A��=0.3mol��B��ƽ��Ũ��ΪA��3����������䣬Ũ��֮�ȵ������ʵ���֮�ȣ��ʴ�ʱA�����ʵ���0.6mol��
| 1 |
| 3 |
473Kʱ�������������䣩��B��ƽ��Ũ��ΪA��2����������䣬Ũ��֮�ȵ������ʵ���֮�ȣ���ƽ��ʱB�����ʵ���Ϊamol����A�����ʵ����仯Ϊ0.5amol���ʣ�0.5mol-0.5amol����2=amol�����a=0.5����ƽ��ʱA�����ʵ���Ϊ0.5��0.5mol=0.25mol���¶����߷�Ӧ���ʼӿ죬��473Kʱ��Ӧ����ƽ���ʱ��С��60s��ͼ��Ϊ��

�ʴ�Ϊ��
 ��
����3�����º���ϡ�����岻Ӱ��ƽ�⣬����1molB����ЧΪ����0.5molA����ԭƽ���Ч��ƽ��ʱB�����ʵ�����ͬΪ0.6mol���μӷ�ӦB�����ʵ���Ϊ1mol-0.6mol=0.4mol����B��ת����Ϊ
| 0.4mol |
| 1mol |
��4������������һ�������б�ʱ�ʾ��ʱ�̵ļ�ʱ��Ӧ���ʣ��ʴ�Ϊ����ʾ��ʱ�̵ļ�ʱ��Ӧ���ʣ�

��ϰ��ϵ�д�
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
 2Z��g����H��0��Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧ��ȡ�Ĵ�ʩ�ǣ� ��
2Z��g����H��0��Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧ��ȡ�Ĵ�ʩ�ǣ� �� N2O4������˵����֤����Ӧ�Ѵ�ƽ��״̬����
N2O4������˵����֤����Ӧ�Ѵ�ƽ��״̬���� 
 ��CH3+Cl2��
��CH3+Cl2��