��Ŀ����
1����ˮ�ɷֵ�ʵ��̽�����£�| ʵ����� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ˮ�μӵ�AgNO3��Һ�� | ���ɰ�ɫ���� | ��ˮ�к���Cl- |
| �� | ����ˮ�μӵ����з�̪��NaOH��Һ�� | ��Һ��ɫ | |
| �� | ȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�� | ����-KI��ֽ����ɫ | ��ˮ�к���Cl2 |
| �� | ����������ˮ�μӵ�Na2CO3��Һ�� | �������� | ��ˮ�к���H+ |
��2��ָ��ʵ��ں�ʵ����е�ʵ��������Һ��ɫ����������ð����
��3��ʵ����С�ʵ�鷽�����ľ�����������ǣ�ȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�ϣ�
��4����ʵ����ʹ�õ��Ǿ��õ���ˮ��ʵ�鷽�����䣬�������٢�ʵ����Ӱ�죮
���� �ټ������������ɰ�ɫAgCl��������֤����ˮ�к���Cl-��
����ˮ�к������ᣬ����NaOH�����кͷ�Ӧ�����д����ᣬ����ǿ�����Ժ�Ư���ԣ�����ʹ��Һ��ɫ���ɸ����кͷ�Ӧ��������ԭ��Ӧ����ͬ������ɫ�����Һ�еμ�����������Һ��������Һ�Ƿ����жϣ�
�ۼ�����ˮ�к���Cl2���ɽ���ˮ���ڵ���-KI��ֽ�ϣ�
����ˮ�����ԣ���Һ�к���H+������þ�ۣ�����������
��1���������������ɰ�ɫAgCl��������֤����ˮ�к���Cl-��
��2����ˮ�����ԣ���Һ�к���H+������Na2CO3��Һ�����ɶ�����̼���壬��ˮ�к������ᣬ����NaOH�����кͷ�Ӧ�����д����ᣬ����ǿ�����Ժ�Ư���ԣ�
��3��������ˮ�к���Cl2���ɽ���ˮ���ڵ���-KI��ֽ�ϣ�
��4����ʵ����ʹ�õ��Ǿ��õ���ˮ���ijɷ������ᣬ��ʵ��ں͢۷ֱ��Ǵ���������������ã�
��� �⣺���ټ������������ɰ�ɫAgCl��������֤����ˮ�к���Cl-��
����ˮ�к������ᣬ����NaOH�����кͷ�Ӧ�����д����ᣬ����ǿ�����Ժ�Ư���ԣ�����ʹ��Һ��ɫ��
�ۼ�����ˮ�к���Cl2���ɽ���ˮ���ڵ���-KI��ֽ��û�������ΪȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�ϣ�
����ˮ�к������ᣬ�ɼ���þ���У����������������Կ���������ð�����ʴ�Ϊ��
| ʵ����� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ��ˮ�к���Cl- | ||
| �� | ��Һ��ɫ | ||
| �� | ȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�� | ||
| �� | �������� |
��2������ˮ�к������ᣬ����NaOH�����кͷ�Ӧ�����д����ᣬ����ǿ�����Ժ�Ư���ԣ���ɹ۲쵽��Һ��ɫ������ˮ�����ԣ���Һ�к���H+������Na2CO3��Һ�����ɶ�����̼���壬�ɹ۲쵽������ð�����ʴ�Ϊ����Һ��ɫ��������ð����
��3��������ˮ�к���Cl2���ɽ���ˮ���ڵ���-KI��ֽ��û�������ΪȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�ϣ�
�ʴ�Ϊ��ȡһСƬKI������ֽ�����ڱ��������Ƭ�ϣ��ýྻ�IJ�����պȡ��ˮ��������ֽ�ϣ�
��4����ʵ����ʹ�õ��Ǿ��õ���ˮ���ijɷ������ᣬ��ʵ��ں͢۷ֱ��Ǵ���������������ã�����ʵ����ʹ�õ��Ǿ��õ���ˮ��ʵ�鷽�����䣬�������٢�ʵ����Ӱ�죬�ʴ�Ϊ���٢ܣ�
���� ����������������Ϊ�����ۺϿ������ʺ����IJⶨ��������ʵ������̽����Ӧ���ʣ����ݷ�Ӧ���ʽ�ʾ��Ӧ����Ĺ��ɵ�̽����ע����ѧ��ʵ��������̽�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��101kPaʱ��1mol������ȫȼ��ʱ���ų������������������ʵ�ȼ���� | |
| B�� | ��ͼ���кͷ�Ӧ����1molˮ����ʱ�ķ�Ӧ�Ƚ��к��� | |
| C�� | ����AlCl3������Һʱ����AlCl3��������ˮ���Լ����ܽ� | |
| D�� | ��ϡ��Һ�У�1molCH3COOH��1mol NaOH��ȫ�к�ʱ�ų�������С��57.3kJ |
| A�� | Na2SiO3 | B�� | BaCl2 | C�� | AgNO3 | D�� | NaAlO2 |
| A�� | 17.1g | B�� | 8.55g | C�� | 34.2g | D�� | 3.4g |
| A�� | 39g | B�� | 59g | C�� | 78g | D�� | 97g |
| A�� | ���³�ѹ�£�17g����-14CH3��������������Ϊ9NA | |
| B�� | 1 L 0.2 mol•L-1��������Һ�к��е�SO42-��Ϊ0.2NA | |
| C�� | 0.1mol N2��������H2��Ӧ��ת�Ƶĵ�����Ϊ0.6NA | |
| D�� | �ö��Ե缫���1L0.1mol•L-1 CuCl2��Һ������0.2NA������ͨ��ʱ��������6.4gͭ |
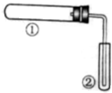 ����ͼװ�ã��г֡�����װ�����ԣ�����ʵ�飬�Т���������֤ʵ���з�Ӧ�������ǣ�������
����ͼװ�ã��г֡�����װ�����ԣ�����ʵ�飬�Т���������֤ʵ���з�Ӧ�������ǣ�������| ����ʵ�� | �������� | |
| �� | ������ˮ�������� | ����ˮð�� |
| �� | ����NH4Cl��Ca��OH��2����� | ��̪��Һ��� |
| �� | NaHCO3 | ����ʯ��ˮ����� |
| A�� | ֻ�Т� | B�� | ֻ�Т� | ||
| C�� | ֻ�Т� | D�� | �����֤ʵ���з�Ӧ���� |
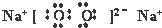 ��
��