��Ŀ����
13����1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S$\frac{\underline{\;��ȼ\;}}{\;}$A+N2��+3CO2��������ƽ�������������У�A�ľ�������Ϊ���Ӿ��壬�����Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊsp��
�ڳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����ΪO��N��C��K��
��2������֪CN-��N2�ṹ���ƣ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ����ԭ������T��Q
��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ3d84s2��Q2+��δ�ɶԵ�������4��
����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln��H2O��6-n]x++x R-H��Rx[CrCln��H2O��6-n]x++x H+����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015mol[CrCln��H2O��6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200mol•L-1 NaOH��Һ25.00ml���������ӵĻ�ѧʽΪ[CrCl ��H2O��5]2+��
���� ��1�������������غ㶨�����жϳ�A�Ļ�ѧʽ��Ȼ���жϾ������ͣ������Թ��ۼ��ķ���ΪCO2�����ݼ۲���Ӷ����жϣ�
�ڽ���Ԫ�ص縺��ԽС������Ԫ��Խ���ã��ǽ���Ԫ�ص縺��Խ�ǽ���Ԫ��Խ���ã�
��2���ٵȵ�����Ľṹ��ͬ�����ݵ����Ľṹ�����������к���1���Ҽ���2���м���
��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ��ٽ�Ϻ�������Ų����ɽ��
�����к����ɵ�H+��Ҫ��NaOH��Һ���ɵó�H+���ʵ��������������x���ٽ��Cr�Ļ��ϼ�+3�ۣ���n��
��� �⣺��1�����ɻ�ѧ����ʽΪS+2KNO3+3C��X+N2��+3CO2�������������غ㶨�ɿ�֪����Ӧǰ��Ԫ�����ࡢԭ�Ӹ�����ȣ���A�Ļ�ѧʽΪK2S���������Ӿ��壻�����Թ��ۼ��ķ���ΪCO2��������̼�ĽṹʽΪO=C=O������2���۲���Ӷԣ�����Cԭ��Ϊsp�ӻ���
�ʴ�Ϊ�����Ӿ��壻sp��
�ڷǽ�����Խǿ����縺��Խǿ���ǽ����ԣ�O��N��C��K����縺��O��N��C��K��
�ʴ�Ϊ��O��N��C��K��
��2����CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ�NiԪ����28��Ԫ�أ�Niԭ�Ӽ۵����Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�ʽΪ1s24s22p63s23d6��3d�ܼ���4�������ӣ�
�ʴ�Ϊ��3d84s2��4��
���к����ɵ�H+��Ũ��Ϊ0.1200mol/L����������Һ25.00mL������Եó�H+�����ʵ���Ϊ0.12mol/L��25.00��10-3L=0.0030mol������x=$\frac{0.0030}{0.0015}$=2��
Cr�Ļ��ϼ�Ϊ+3�ۣ����[CrCln��H20��6-n]x+����3-n=2�����Ե�֪n=1�����������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+��
�ʴ�Ϊ��[CrCl ��H2O��5]2+��
���� ���⿼���˵縺�ԡ��������͵��жϡ��ӻ�������ȵ��ӡ�Ԫ�����ڱ�����������Ų��ȣ�ע����յȵ�����ԭ�������ݡ����ڱ��мȴ���ͬһ������λ��ͬһ�塱ȷ��Ԫ���ǹؼ�����Ŀ�Ѷ��еȣ��״�����Feԭ��ʧȥ4s�ϵ�2�������γ�Fe 2+��������ʧ3d�ϵĵ��ӣ�

| A�� | 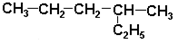 2-�һ����� 2-�һ����� | B�� |  2-��-2-�ȱ��� 2-��-2-�ȱ��� | ||
| C�� | 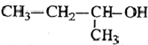 2-��-1-���� 2-��-1-���� | D�� |  2-��-3-��Ȳ 2-��-3-��Ȳ |
| A�� | ������Ӧ���ӳɷ�Ӧ | B�� | ��ԭ��Ӧ��������Ӧ | ||
| C�� | ������Ӧ��ȡ����Ӧ | D�� | �ӳɷ�Ӧ��ˮ�ⷴӦ |
| A�� | 12 | B�� | 32 | C�� | 60 | D�� | 80 |
| A�� | SO2��O2��SO3������������ٸı� | |
| B�� | c��SO2����c��O2����c��SO3��=2��1��2 | |
| C�� | SO2��O2�����ʵ���֮����SO3�����ʵ���2�� | |
| D�� | ��λʱ����ÿ����2molSO2��ͬʱ����1molO2 |
| A�� | ʵ��װ�ñ��¡�����Ч���� | |
| B�� | ��ȡ�����Һ�����ʱ���Ӷ��� | |
| C�� | �ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��� | |
| D�� | ���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� |
| A�� | ��Ӧ������I-Ũ���й� | B�� | IO-Ҳ�Ǹ÷�Ӧ�Ĵ��� | ||
| C�� | �û�ѧ��Ӧ�����ɷ�Ӧ�ھ��� | D�� | v��H2O2��=v��H2O��=v��O2�� |
 ij��ȤС����̽��������̼������������Һ��Ӧ��ʵ���У�������ͼ��ʾ��ʵ�飮
ij��ȤС����̽��������̼������������Һ��Ӧ��ʵ���У�������ͼ��ʾ��ʵ�飮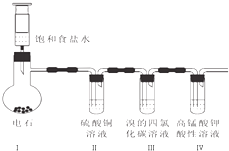 ʵ��������Ȳ�����������ʵ�װ����ͼ��
ʵ��������Ȳ�����������ʵ�װ����ͼ��