��Ŀ����
����Ŀ��(1)�������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�塣��������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
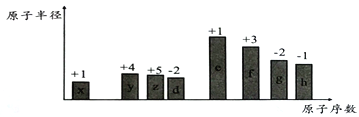
����ͼ��һ����������ȫȼ������CO2��1molH2O(l)�����е������仯ͼ������ͼ�е�������������+�������� ___��
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___��
�۶�����(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ___��
(2)��˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵�����ЧӦ��ͬ�������ø�˹���ɻش��������⣺
����֪��H2O(g)�TH2O(l) ��H1=Q1kJ/mol
C2H5OH(g)�TC2H5OH(l) ��H2=Q2kJ/mol
C2H5OH(g)+3O2(g)�T2CO2(g)+3H2O(g) ��H3=Q3kJ/mol
��ʹ23gҺ̬��ˮ�ƾ���ȫȼ�գ����ָ������£������������зų�������Ϊ___kJ��
��̼(s)��������Ӧ�����ʱ������COͬʱ����������CO2�������ͨ��ʵ��ֱ�Ӳ�÷�Ӧ��C(s)+![]() O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
O2(g)�TCO(g)����H.�������ʵ�顢���ø�˹���ɼ�����÷�Ӧ����H������ʱ��Ҫ��õ�ʵ��������___��
���𰸡� C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol 1:3 1.5Q10.5Q2+0.5Q3 ̼��һ����̼�ı�ȼ����
��������
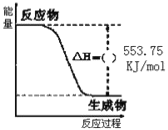
(1)����ͼ��֪����Ӧ����������������������������
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol��
�������Ȼ�ѧ����ʽ��ϻ���������ʵ����ͷ�����ʽ����õ������Ѻͱ������ʵ���֮�ȣ�
(2)�����ݸ�˹���ɼ���ɵã�
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ���
(1)����ͼ��֪����Ӧ�����������������������������÷�ӦΪ��Ӧ���ȣ���HΪ�������ʴ�Ϊ����
����ͼ���֪��������ȫȼ������1molˮ���ʱ���H=553.75kJ/mol����Ӧ���Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol���ʴ�Ϊ��C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)��H=2215kJ/mol��
��1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ��������1mol��������ж��������ʵ���x���������ʵ���Ϊ1x�����Ȼ�ѧ����ʽ��֪����ȼ�շ���2215 (1x) kJ��������ɵù�ϵʽ16451455x=2215 (1x)�����x=0.75�����ϱ������ʵ���Ϊ0.25mol����������б���Ͷ��������ʵ���֮��=0.25:0.75=1:3���ʴ�Ϊ��1:3��
(2)�ٽ���֪�Ȼ�ѧ����ʽ���α��Ϊ�١��ڡ��ۣ��ɸ�˹���ɿ�֪������+����3���Ȼ�ѧ����ʽC2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l) ������H1=(3Q1Q2+Q3)kJ/mol����ʹ23gҺ̬��ˮ�ƾ����ʵ���Ϊ0.5mol����ȫȼ�գ����ָ������£������������зų�������Ϊ(1.5Q10.5Q2+0.5Q3)kJ���ʴ�Ϊ��1.5Q10.5Q2+0.5Q3��
�����ʵ�顢���ø�˹���ɼ���C(s)+ ![]() O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�
O2(g)�TCO(g)����H����Ҫ֪��̼��һ����̼��ȼ���Ȳ��ܼ���õ����ʴ�Ϊ��̼��һ����̼�ı�ȼ���ȡ�

����Ŀ���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״���
��1����֪����H2(g)��1/2O2(g)![]() H2O(l) ��H1����285.8 kJ/mol��
H2O(l) ��H1����285.8 kJ/mol��
��CO (g)��1/2O2 (g)![]() CO2 (g) ��H2=��283kJ/mol
CO2 (g) ��H2=��283kJ/mol
��CH3OH(g)��3/2O2(g)![]() CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
CO2(g)��2H2O(l) ��H3����764.6 kJ/mol
��ҵ�Ʊ��״��Ŀ��淴Ӧ�Ȼ�ѧ����ʽΪ_______________________________��
��2�����º��������£�������������˵��������Ӧ�Ѵ�ƽ��״̬����__________��
A����λʱ��������n mol CO��ͬʱ����2n mol H2 B����(H2)����2��(CH3OH)��
C��������������ܶȱ��ֲ��� D�������������ѹǿ���ֲ���
��3��ij��ѧ�о���ѧϰС��ģ�ҵ�ϳɼ״��ķ�Ӧ�����ݻ��̶�Ϊ2L���ܱ������ڳ���1 molCO�� 2 molH2��������ʴ�����������Ժ��Բ��ƣ�����250��C��ʼ��Ӧ��CO���ʵ�����ʱ��仯���£�
��Ӧʱ��/min | 0 | 5 | 10 | 15 | 20 | 25 |
n��CO��/mol | 1.00 | 0.79 | 0.63 | 0.54 | 0.50 | 0.50 |
��ӷ�Ӧ��ʼ��20minʱ��������H2��=________�����¶���ƽ�ⳣ��K��_______��
��4�������������250 ����ʼ��Ӧ��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
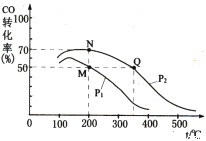
��M��N��Q�����ƽ�ⳣ��KM��KN��KQ�Ĵ�С��ϵΪ___________________________��
����M�㵽N��ı�����������_________��
A�������¶� B������ѹǿ
C�����ø��õĴ��� D��ͨ������CO
��5��25��ʱ��ϡ����Ϊ�������Һ�Ƴɼ״�ȼ�ϵ�أ����ĵ缫����ʽΪ_________________________��