��Ŀ����
����Ŀ����̼����(2 Na2CO3��3 H2O2)�㷺���ڻ�������ֽ����֯��ʳƷ����ҵ��һ����â��(Na2SO4��10 H2 O)��H2O2��Ϊԭ���Ʊ���̼���ƵĹ����������£�
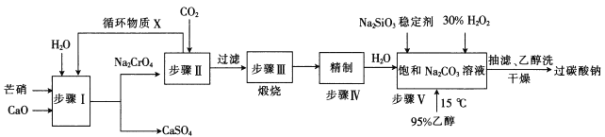
��֪2CrO42-+2H+=Cr2O72-+H2O �� pHС��5ʱ��������Cr2O72-��ʽ���ڣ�pH����8.5ʱ��������CrO42-��ʽ���ڡ�
�ش��������⣺
(1)Na2CrO4��CrԪ�صĻ��ϼ�Ϊ___
(2)Ksp(CaCrO4)___(����������������)Ksp(CaSO4)��
(3)������ѭ������XΪ___(�ѧʽ)��
(4)����II�з�����Ӧ�Ļ�ѧ����ʽΪ_________
(5)����IһIII��Ϊ���Ƶô���ӻ��������ĽǶȿ������ܵIJ���֮����___
(6)����V�ϳ�ʱ������95%���Ҵ���Ŀ����___
(7)�ⶨ��Ʒ��������ʵ�鲽�����£�ȷ��ȡmg��Ʒ���������ܽ����c mol��L��1��KMnO4����Һ�ζ����յ㣬����KMnO4����ҺV mL��
�ٵζ����յ�ʱ����Һ��___(������ɫ������dz��ɫ��)��
�ڹ�������Ļ�������ָ�������ﵥ���ô������ֽ�ʱ�ų���������������Ʒ������֮�ȡ���ʵ���õIJ�Ʒ�л�����Ϊ___(�г��������ʽ)��
���𰸡�+6 > Na2Cr2O7 ![]() ���۸��ж�������ɻ�����Ⱦ ��С��̼���ɵ��ܽ�ȣ���߲��� dz��ɫ
���۸��ж�������ɻ�����Ⱦ ��С��̼���ɵ��ܽ�ȣ���߲��� dz��ɫ ![]()
��������
��â����Na2SO410H2O����H2O2��Ϊԭ���Ʊ���̼���ƣ�â������ˮ�������Ʒ�Ӧ�õ�����ƣ��������Ʒ�Ӧ�õ�����ƺ����ƣ���Һ��ͨ�������̼��Ӧ����̼�����ƾ�����ظ����ƣ����˵õ�̼���������շֽ�����̼���ƣ�����ˮ���Ƶõ�̼������Һ������������ȶ�����30%�Ĺ������⡢����95%���Ҵ��ͱ���̼������Һ��Ӧ�õ���̼���ƾ��壬�����Ҵ�ϴ�Ӹ���õ���̼���ƣ�
��1��Na2CrO4����Ԫ�ػ��ϼ�+1�ۣ���Ԫ��-2�ۣ����ϼ۴�����Ϊ0����õ�CrԪ�صĻ��ϼۣ�
��2���������̿�֪�����Na2CrO4����CaSO4��˵����Ӧ������ܵķ�����У�
��3����֪2CrO42-+2H+Cr2O72-+H2O��pHС��5ʱ��������Cr2O72-��ʽ���ڣ�pH����8.5ʱ��������CrO42-��ʽ���ڣ�����Һ��ƽ��������У�
��4��������з�����Ӧ�Ķ�����̼ͨ���������Һ�з�����Ӧ�����ظ����ƺ�̼�����ƾ��壻
��5�����۸��ж�������ɻ�����Ⱦ��
��6������V�ϳ�ʱ������̼������Һ�У���������ơ�30%�Ĺ������⡢95%���Ҵ��õ���̼���ƾ��壻
��7���ٵζ��յ��ǵ������һ�θ��������Һ����Һ��ɫ��Ϊ�Ϻ�ɫ�Ұ���Ӳ��䣬˵����Ӧ�ﵽ�յ㣻
�ڹ�������Ļ�������ָ�������ﵥ���ô������ֽ�ʱ�ų���������������Ʒ������֮�ȣ��ݴ�д��ʵ���õIJ�Ʒ�л�������
��1��Na2CrO4����Ԫ�ػ��ϼ�+1�ۣ���Ԫ��-2�ۣ����ϼ۴�����Ϊ0����õ�CrԪ�صĻ��ϼ�Ϊ+6�ۣ�
�ʴ�Ϊ��+6��
��2���������̿�֪�����Na2CrO4����CaSO4��˵����Ӧ������ܵķ�����У�֤��Ksp��CaCrO4����Ksp��CaSO4����
�ʴ�Ϊ������
��3����֪2CrO42-+2H+Cr2O72-+H2O��pHС��5ʱ��������Cr2O72-��ʽ���ڣ�pH����8.5ʱ��������CrO42-��ʽ���ڣ�����Һ��ƽ��������У�������ѭ������XΪ��Na2Cr2O4��
�ʴ�Ϊ��Na2Cr2O4��
��4��������з�����Ӧ�Ķ�����̼ͨ���������Һ�з�����Ӧ�����ظ����ƺ�̼�����ƾ��壬��Ӧ�Ļ�ѧ����ʽΪ��2Na2CrO4+2CO2+H2ONa2Cr2O7+2NaHCO3����
�ʴ�Ϊ��2Na2CrO4+2CO2+H2ONa2Cr2O7+2NaHCO3����
��5�������-����Ϊ���Ƶô���ӻ��������ĽǶȿ������۸��ж�������ɻ�����Ⱦ��
�ʴ�Ϊ�����۸��ж�������ɻ�����Ⱦ��
��6������V�ϳ�ʱ������95%���Ҵ���Ŀ���ǣ���С��̼���Ƶ��ܽ�ȣ���߲��ʣ�
�ʴ�Ϊ����С��̼���Ƶ��ܽ�ȣ���߲��ʣ�
��7���ٵζ��յ��ǵ������һ�θ��������Һ����Һ��ɫ��Ϊ�Ϻ�ɫ�Ұ���Ӳ��䣬֤����Ӧ�ﵽ��Ӧ�յ㣬
�ʴ�Ϊ��dz��ɫ��
��ȷ��ȡmg��Ʒ���������ܽ����cmolL-1��KMnO4����Һ�ζ����յ㣬����KMnO4����ҺVmL����ӦΪ��5H2O2+3H2SO4+2KMnO4=K2SO4+2MnSO4+8H2O+5O2����n��H2O2��=![]() mol=2.5��10-3cVmol��2H2O2=2H2O+O2�����ֽ�������������=2.5��10-3cVmol��32g/mol��
mol=2.5��10-3cVmol��2H2O2=2H2O+O2�����ֽ�������������=2.5��10-3cVmol��32g/mol��![]() = 0.04cV g����������Ļ�������ָ�������ﵥ���ô������ֽ�ʱ�ų���������������Ʒ������֮�� =
= 0.04cV g����������Ļ�������ָ�������ﵥ���ô������ֽ�ʱ�ų���������������Ʒ������֮�� = ![]() ��100% =
��100% = ![]() ��
��
�ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��I.�������Ƶ�������Ӧ:2Na2SO3 (aq) +O2(aq)=2Na2SO4(aq) H=x kJ/mol���䷴Ӧ�������ܽ���Ũ��Ӱ��,��Ϊ��������ƶ���������Ρ�
��1����֪O2(g) ![]() O2(aq) H=y kJ/mol��Na2SO3 ��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ___________________��
O2(aq) H=y kJ/mol��Na2SO3 ��Һ��O2(g)��Ӧ���Ȼ�ѧ����ʽΪ___________________��
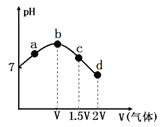
��2��291.5 Kʱ,1.0 L��Һ��Na2SO3��ʼ���ֱ�Ϊ4��6��8��12 mmol,�ܽ���Ũ�ȳ�ʼֵΪ9.60 mg/L,ÿ5 s��¼�ܽ���Ũ��,ʵ������ͼ��ʾ����Na2SO3��ʼ��Ϊ12 mmol,����20 s�ܽ���Ũ�Ƚ�Ϊ6.40 mg/L,��0��20s��Na2SO3��ƽ����Ӧ����Ϊ_______mol/(L��s)��
��3��Ϊȷ��ƶ�������ʷ���v=k��ca(SO32-)��cb(O2)�е�a��b��ֵ(ȡ����),����ʵ�����ݡ�
c(Na2SO3)��103 | 3.65 | 5.65 | 7.65 | 11.65 |
v��106 | 10.2 | 24.4 | 44.7 | 103.6 |
�ٵ��ܽ���Ũ��Ϊ4.0 mg/Lʱ,c(SO32-)��������ֵ��ϵ���(��)��ʾ����a=____��
�ڵ��ܽ���Ũ��С��4.0mg/Lʱ,ͼ�����߽�Ϊֱ��,Na2SO3�����������ܽ���Ũ����,��b=_______��
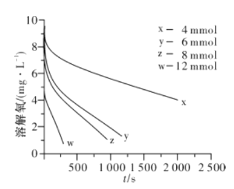
��4�������β�ͬ�¶ȵ����ʳ���֮�����(��)��ʾ����֪![]() ,RΪ������Ea(������)_____ (���������)Ea(ƶ����)��
,RΪ������Ea(������)_____ (���������)Ea(ƶ����)��
��Ӧ�� | ���ʷ��� |
|
������ | v=k��c (SO32-)��c (O2) | 1.47 |
ƶ���� | v=k��ca (SO32-)��cb(O2) | 2.59 |
II. ��5�����ݻ��̶����ܱ�������,��ʼ����0.2 mol SO2��0.1 mol O2,��Ӧ��ϵ��ʼ��ѹǿ0.1MPa����Ӧ��һ���¶��´ﵽƽ��ʱSO2��ת����Ϊ90%���÷�Ӧ��ѹǿƽ�ⳣ��Kp=________ ( ��ѹ=��ѹ�����ʵ�������)(д��λ)��
��6������ԭ���ԭ��,Ҳ����SO2��O2���Ʊ�����,�õ���ö�ײ������缫����д���õ�ظ�����Ӧʽ_________________________��
����Ŀ�������йػ�ѧʵ�����������ͽ��۾�Ϊ��ȷ����
ѡ�� | ���� | ���� | ���� |
A | Mg2+��Cu2+�������Һ�е�������NaOH��Һ | ������ɫ���� | ��ͬ�¶��£��ܶȻ����� Ksp��Mg(OH)2�ݣ�Ksp��Cu(OH)2�� |
B | ����ʢ��(NH4)2CO3������Թܣ������Թܿڷ���ʪ��ĺ�ɫʯ����ֽ | ��ֽ���� | (NH4)2CO3�Լ��� |
C | �����£����Ũ�ȡ��������Na2CO3��NaHCO3��Һ�еμӵ����ķ�̪��Һ | ̼������Һ�к�ɫ���� | ������ˮ�ⳣ��Kh: CO32-��HCO3- |
D | ��������Һ�еμ�����Na2CO3��ĩ | ������ð�� | ����֤������:̼����� |
A.AB.BC.CD.D