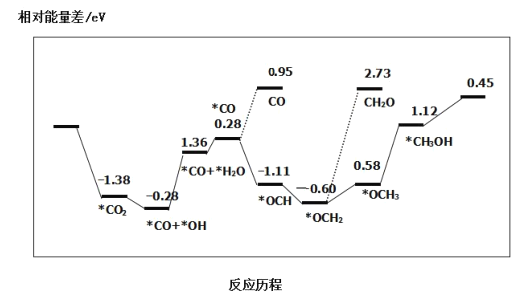
��Ŀ����
����Ŀ��������Ԫ�� X��Y��Z��W��R��T ��ԭ�������������� X �������������������������ȣ� X��W λ��ͬ�塣Y �� Z �ļ۵�����֮�͵��� R �� T ������������֮�ͣ�������Ԫ����������� Z2TY3 �� ZRY2���ں��������εĻ����Һ�еμ����ᣬ������ɫ���������ʵ�������������Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
A.X��Y��Z ������ͬ������һ�����ӻ�������
B.���ʵ��۵㣺Y < Z < T
C.�����ӵİ뾶��r(Y)> r(W)> r(Z)
D.Z��R �ֱ��� Y �γɵĻ������ж�ֻ�����Ӽ�
���𰸡�B
��������
������Ԫ��X��Y��Z��W��R��T ��ԭ��������������X �������������������������ȣ���XΪBeԪ�أ�X��Wλ��ͬ�壬��WΪMgԪ�أ�������Ԫ�����������Z2TY3��ZRY2���ں��������εĻ����Һ�еμ����ᣬ������ɫ���������ʵ�������������Ĺ�ϵ��ͼ��ʾ������ͼʾ��֪���ܽ�İ�ɫ����Ϊ������������ZRY2ΪNaAlO2��ZΪNaԪ�أ�RΪAl��YΪOԪ�أ�Y��Z�ļ۵�����֮�͵���R��T������������֮�ͣ���T������������Ϊ6+1-3=4����TΪSiԪ�ء�
���ݷ�����֪��XΪBe��YΪO��ZΪNa��WΪMg��RΪAl��TΪSiԪ�ء�
A��X��Y��Z ����ͬ������һ�����ӻ������У�������ΪNa2BeO2����A����
B�����Ƿ��Ӿ��壬���ǽ������塢����ԭ�Ӿ��壬���ʵ��۵㣺Y < Z < T����B��ȷ��
C�������ӡ�þ���Ӻ������Ӻ�����ͬ���Ӳ������˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶r��W����r��Z����r��Y������C����
D��Na2O2�в�ֻ�����Ӽ���������֮���γɷǼ��Թ��ۼ�����D����
��ѡB��

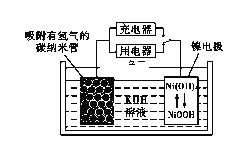
����Ŀ��ij����(Ni)�ϴ�������Ҫ����Ni��������Al��Al2O3��Fe�������������ᡢ������ʡ����ֽ�����������Ksp����ֵ���±���ʾ��
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp����ֵ | 10-17 | 10-39 | 10-34 | 10-15 |
���ú����ϴ����Ʊ�NiSO4��7H2O���壬������ͼ���£�
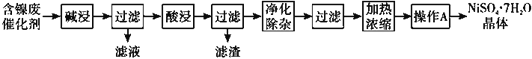
�ش��������⣺
��1���������ʱ������Ӧ�����ӷ���ʽΪ2A1+2OH-+2H2O=2AlO2��+3H2����_________��
��2�����������ʹ�õ���Ϊ_____________��
��3�������������������H2O2��Һ����������__________________________��Ȼ�����pHʹ��Һ����Ԫ��ǡ����ȫ��������ʱ��pHΪ____________������1λС������
��4��������A��Ϊ_____�����ˡ�ϴ�ӡ�������ò�Ʒ��
��5��NiSO4��NaOH��Һ�пɱ�NaClO����ΪNiOOH���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
��6��NiOOH����Ϊ�����صĵ缫���ϣ��õ�صĹ���ԭ������ͼ��ʾ������ʱ�������ĵ缫��ӦʽΪ____________��