��Ŀ����
��֪����������K2SO4��MgSO4��2CaSO4��ˮ�д�������ƽ�⣺
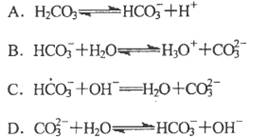
K2SO4��MgSO4��2CaSO4(s) 2Ca2++2K++Mg2++4SO42������ͬ�¶��£�K+�Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ��ʾ��������˵���������
2Ca2++2K++Mg2++4SO42������ͬ�¶��£�K+�Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ��ʾ��������˵���������
| A�������ϵ�м��뱥��NaOH��Һ���ܽ�ƽ�������ƶ� |
| B�������ϵ�м��뱥��̼������Һ���ܽ�ƽ�������ƶ� |
| C�������¶ȣ���Ӧ��������ƽ��������Ӧ�����ƶ� |
| D����ƽ���Ksp= c(Ca2+) ��c(K+)��c(Mg2+)��c(SO42��) |
D
�������������A�������ϵ�м��뱥��NaOH��Һ��OH?��Mg2+�������Mg(OH)2������ʹ�ܽ�ƽ�������ƶ�����ȷ��B�������ϵ�м��뱥��̼������Һ��CO32?���Ca2+��Mg2+������CaCO3��MgCO3������ʹ�ܽ�ƽ�������ƶ�����ȷ��C������ͼ����Կ������¶����ߣ��ܽ�ƽ�������ƶ�����ȷ��D����ƽ���Ksp= c2(Ca2+) ��c2(K+)��c(Mg2+)��c4(SO42��)������
���㣺���⿼������ܽ�ƽ���Ksp�ı���ʽ��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д������£��� 0��01 mol��L��1NaOH��Һ�ζ� 20��00 mL 0��01 mol��L��1 CH3COOH��Һ�����õζ�������ͼ������˵����ȷ����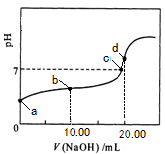
| A��a���Ӧ��Һ��pH=2 |
| B��b���Ӧ����Һ�У�c(OH��)+ c(CH3COO��) = c(Na+)+ c(H+) |
| C��c���ʾNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ |
| D��d���Ӧ����Һ�У�ˮ�ĵ���̶�С��ͬ���´�ˮ�ĵ���̶� |
���л�ѧʵ����ʵ������ͻ���۶���ȷ���ǣ� ��
| A��ȡ������ҺX�������м�������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2�� |
| B�����Ҵ��������ἰpH=0��H2SO4���ȿ��������������ñ��͵�Na2CO3��Һ����ϴȥ���к��е��Ҵ������ᣬ˵������������Na2CO3��Һ�е��ܽ�Ⱥ�С |
| C����Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ������������ɫ������˵��Ksp[Cu(OH)2] ��Ksp[Mg(OH)2] |
| D������0.10mol/L NaCl��Һ����������ƿ�Ŀ̶��߶��ݣ�������ҺŨ��ƫ�� |
�����£�ij��Һ����ˮ�����c(H+)=1��10��13mol/L������Һ������
�ٶ�������ˮ��Һ���Ȼ��ˮ��Һ��̼����ˮ��Һ����������ˮ��Һ
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
�������ʵ�ˮ��Һ�У�����ˮ�����⣬�������������ӵ���( )��
| A��HCI | B��NH4NO3 | C��Na2S | D��HClO |
�й�ˮ�ĵ���ƽ���˵����ȷ����(����)
| A��ˮ�����ӻ�ͨ����KW����ʾ�����¶ȱ仯���仯��ֻ�����ڴ�ˮ����ϡ��������ֵ���� |
| B���ڴ�ˮ�м������������ˮ�ĵ��룬�Ӵ����ٽ�ˮ�ĵ��� |
| C��������ˮ��ˮ�ĵ���ƽ���û��Ӱ�� |
| D���ڴ�ˮ�м����������������ˮ�ĵ���ƽ���������Ӱ�� |
ij�¶��£���һ�����0.1 mol��L��1�Ĵ�����Һ����μ����Ũ�ȵ�NaOH��Һ����Һ��pOH��pOH����lg[OH��]����pH�ı仯��ϵ��ͼ��ʾ��������
| A��M����ʾ��Һ�ĵ�������ǿ��Q�� |
| B��N����ʾ��Һ��c��CH3COO����>c��Na���� |
| C��M���N����ʾ��Һ��ˮ�ĵ���̶���ͬ |
| D��Q������NaOH��Һ��������ڴ�����Һ����� |
ij�¶�ʱ,Ag2SO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ�����¶���,����˵����ȷ����(����)
A�����д���S ����Һ�п϶�������Ag+ ����Һ�п϶�������Ag+ |
| B��0.02 mol��L-1��AgNO3��Һ��0.02 mol��L-1��Na2SO4��Һ�������ϲ������ɳ��� |
| C��Ag2SO4���ܶȻ�����(Ksp)Ϊ1��10-3 |
| D��a���ʾAg2SO4�IJ�������Һ,��������ʹ��Һ��a��䵽b�� |