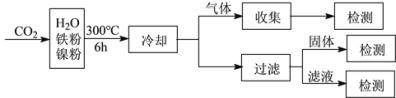
��Ŀ����
����Ŀ����һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�H����NH4+��K����Mg2����Cu2����Al3����NO3-��CO32-��SO42-����ȡ����100 mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�������AgNO3��Һ���а�ɫ����������
�ڵڶ��ݼ�������BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99 g��
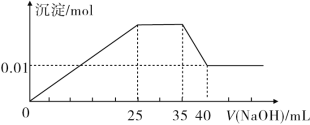
�۵�������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ��ʾ��
��������ʵ�飬����������⣺
��1��ԭ��Һ��һ�������ڵ�������______________��
��2��25��35�η�����Ӧ�����ӷ���ʽΪ__________��
��3��35��40�η�����Ӧ�����ӷ���ʽΪ__________��
��4��ʵ�����ӵ�NaOH��Ũ��Ϊ________��
��5��ԭ��Һ��NO3-�����ʵ���Ϊn(NO3-)�������ṩ��ͼ������ݣ��Լ���n(NO3-)��ȡֵ��ΧΪ________��
���𰸡�Cu2����CO32-��H�� NH4+��OH��===NH3��H2O Al��OH��3��OH��===AlO2-+2H2O 2 mol��L��1 n(NO3-)��0.01 mol
��������
������ɫ��Һ��֪������ɫ�����Ӳ��ܴ��ڣ�һ������Cu2����
�ٵ�һ�ݼ�����AgNO3��Һ���а�ɫ���������������ƶ�һ����CO32-��SO42-���������е�һ�֣�
�ڵڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99g�����ƶ�һ����CO32-��SO42-���������е�һ�֣�
�۵�������εμ�NaOH��Һ����ͼ��֪����ʼ��������������Һʱ�����г������ɣ�˵��������H+���������ʱ���μ�����������Һ���������ܽ⣬˵������笠����ӡ�����������������ʱ���������ܽ⣬�����Һ����Ȼ�г������ƶ�һ������Mg2����Al3����Mg2����Al3����̼������Ӳ����棬������Һ��һ������̼������ӣ�������������ӣ����ͼ���Լ�����غ���
��1�����ᱵ�����ʵ�����6.99g��233g/mol��0.03mol����ÿһ����Һ������������ʵ�����0.03mol������ͼ���֪��笠����ӷ�Ӧ������������10mL���ܽ�����������������������5mL���������������������������15mL�����Գ���þ������������������25mL��15mL��10mL�����յõ�������þ������0.01mol������ÿһ����Һ��þ������0.01mol����þ���ӷ�Ӧ������������0.02mol�����������Ƶ�Ũ����0.02mol��0.01L��2mol/L������ÿһ����Һ��笠������ʵ�����0.01L��2mol/L��0.02mol��������������Ӧ������������2mol/L��0.005L��0.01mol����ÿһ����Һ����������0.01mol�����ݵ���غ��֪��Һ��һ��������������ӣ��������Ӳ���ȷ�������Ը������Ϸ�����֪ԭ��Һ��һ��������Cu2����CO32-��H����
��2���������Ϸ�����֪25��35�η�����Ӧ�����ӷ���ʽΪNH4+��OH��=NH3��H2O��
��3���������Ϸ�����֪35��40�η�����Ӧ�����ӷ���ʽΪAl��OH��3��OH��=AlO2-+2H2O��
��4���������Ϸ�����֪ʵ�����ӵ�NaOH��Ũ��Ϊ2 mol��L��1��
��5��������ڼ����ӣ������ˮ�ĵ��룬���ݵ���غ��֪ÿһ����Һ��NO3-�����ʵ���n(NO3-)��0.02mol+0.01mol��2+0.01mol��3��0.03mol��2��0.01mol��

����Ŀ����1����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-283.0kJ��mol-1��-726.5kJ��mol-1����ô��̫���ֽܷ�10molˮ���ĵ�������____________kJ��
��2���ɺϳ��������ΪH2��CO��������CO2��ֱ���Ʊ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
��i��CO(g) + 2H2(g) = CH3OH(g) ��H1 = -90.1kJmol-1
��ii��CO2(g) + 3H2(g) = CH3OH(g) + H2O(g) ��H2 = -49.0kJmol-1
ˮú���任��Ӧ��
��iii��CO(g) + H2O(g) = CO2(g) + H2 (g) ��H3 = -41.1kJmol-1
�����Ѻϳɷ�Ӧ��
��iV��2 CH3OH(g) = CH3OCH3(g) + H2O(g) ��H4 = -24.5kJmol-1
����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ____________��
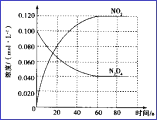
��3�����ݻ�Ϊ1.00L�������У�ͨ��һ����N2O4������N2O4(g) ![]() 2NO2(g), 100��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60sʱ�Σ�v(N2O4)=_______________________��
2NO2(g), 100��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60sʱ�Σ�v(N2O4)=_______________________��
��4����֪��Ӧ2HI��g��=H2(g) + I2(g)�Ħ�H= +11kJ��mol-1��1molH2��g����1mol I2��g�������л�ѧ������ʱ�ֱ���Ҫ����436kJ��151kJ����������1molHI��g�������л�ѧ������ʱ�����յ�����Ϊ___________kJ����716Kʱ�����������е⻯������ʵ�������x(HI)�뷴Ӧʱ��t�Ĺ�ϵ���±�
t/min | 0 | 20 | 40 | 60 | 80 | 120 |
x(HI) | 1 | 0.91 | 0.85 | 0.815 | 0.795 | 0.784 |
������Ӧ�У�����Ӧ����Ϊv��= k����x2(HI)���淴Ӧ����Ϊv��=k����x(H2)��x(I2)������k����k��Ϊ���ʳ����� ��k�� = 0.0027min-1����t=40minʱ��v��=_______min-1