��Ŀ����
����Ŀ��������ЧӦ����ȫ���ע�Ļ�������֮һ��CO2��Ŀǰ�����к�����ߵ�һ���������塣��ˣ����ƺ�����CO2�ǽ������ЧӦ����Ч;����
��1�����д�ʩ�У������ڽ��ʹ�����CO2Ũ�ȵ��У�_________��������ĸ��
a�����ٻ�ʯȼ�ϵ�ʹ�� b��ֲ�����֣�����ֲ�����
c�����ý��ܼ��� d������̫���ܡ�����
��2����CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
a��6CO2 + 6H2O![]() C6H12O6 b��CO2 + 3H2
C6H12O6 b��CO2 + 3H2![]() CH3OH +H2O
CH3OH +H2O
c��CO2 + CH4![]() CH3COOH d��2CO2 + 6H2
CH3COOH d��2CO2 + 6H2![]() CH2==CH2 + 4H2O
CH2==CH2 + 4H2O
���Ϸ�Ӧ�У�����ܵ���_____________��ԭ����������ߵ���____________��
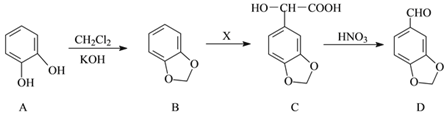
��3�����ױ���ij����������CO2���⻯�Ƽ�����о��������£�
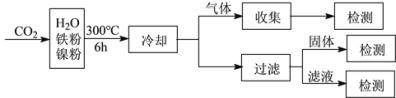
��Ӧ�����������м�CH4��H2����Һ�м�HCOOH�������м����ۺ�Fe3O4��
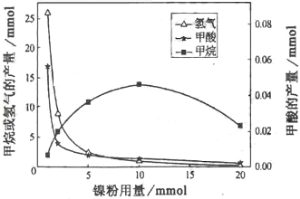
CH4��HCOOH��H2�IJ��������������Ĺ�ϵ����ͼ��ʾ�����ı����������������������䣩��
�о���Ա����ʵ�����ó����ۣ�
HCOOH��CO2ת��ΪCH4���м��壬����CO2![]() HCOOH
HCOOH![]() CH4
CH4
��д������H2�ķ�Ӧ����ʽ_______________________________________��
����ͼ��֪��������_______��������ĸ��
a����Ӧ��Ĵ���
b����Ӧ��Ĵ���
c����Ӧ���Ĵ���
d�����Ǵ���
��������������1mmol���ӵ�10mmol����Ӧ���ʵı仯�����__________��������ĸ��
a����Ӧ����������ӣ���Ӧ������ʲ���
b����Ӧ������ʲ��䣬��Ӧ�����������
c����Ӧ�������ʾ�����
d����Ӧ�������ʾ����ӣ��ҷ�Ӧ����������ӵÿ�
e����Ӧ�������ʾ����ӣ��ҷ�Ӧ����������ӵÿ�
f����Ӧ������ʼ�С����Ӧ�����������
���𰸡�abcdac![]() ce
ce
��������
��1��a�����ٻ�ʯȼ�ϵ�ʹ�ã����Լ�����������ŷŶ�����̼��������ȷ��b��ֲ�����֣�����ֲ�����������ͨ������������ղ��ֶ�����̼��������ȷ��c�����ý��ܼ������ɼ��ٻ�ʯȼ�ϵ����ã�Ҳ�ܼ��ٶ�����̼���ŷţ�������ȷ��d������̫���ܡ����ܣ����ٻ�ʯȼ�ϵ�ʹ�ã����ٶ�����̼���ŷţ�������ȷ����ѡabcd����2�������������Ȼ����̼ѭ���ķ�Ӧ������Ҫ����Ϊ�ṩ��Դ��������ã���ѡa��c�ǻ��Ϸ�Ӧ������ԭ�Ӷ�����˲��������������ߣ���ѡc����3���ܱ������и���������ˮ�������ɴ˿��Բ���H2����3Fe+4H2O![]() Fe3O4+4H2����Ӧǰ�����Ni�ۣ���ͼ���������۵�������ͬ�����ߵ��ߵ����Ʋ�ͬ������Ӧ�Ŀ�����ͬ�������������������ѡc����ͼ����Է���������Ni�ļ��룬H2��CH4�������ǿ��ټ�С�ģ�����Ӧ���������ӵģ����ڼ���ļ������仯���죬��˵����ӦII���ʸ��죬��ѡe��
Fe3O4+4H2����Ӧǰ�����Ni�ۣ���ͼ���������۵�������ͬ�����ߵ��ߵ����Ʋ�ͬ������Ӧ�Ŀ�����ͬ�������������������ѡc����ͼ����Է���������Ni�ļ��룬H2��CH4�������ǿ��ټ�С�ģ�����Ӧ���������ӵģ����ڼ���ļ������仯���죬��˵����ӦII���ʸ��죬��ѡe��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����и��������У�X����Ҫ���ʣ�Y���������ʣ�Z��Ϊ��ȥ������Ҫ������Լ������������Լ���ȷ������� (����)
A | B | C | D | |
X | FeCl2��Һ | FeCl3��Һ | Fe | Na2SO4��Һ |
Y | FeCl3 | CuCl2 | Al | Na2CO3 |
Z | Cu | Fe | NaOH��Һ | BaCl2��Һ |
A. A B. B C. C D. D