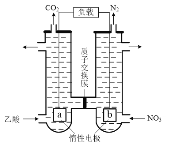
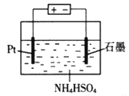
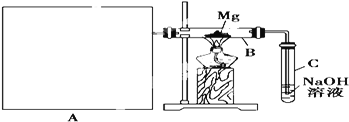
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ¥ΉΥαΚΆ“ΜΥ°ΚœΑ± «÷–―ßΜ·―ß÷–≥ΘΦϊΒΡ»θΒγΫβ÷ ΓΘ
Θ®1Θ©≥ΘΈ¬œ¬Θ§Ρ≥―–ΨΩ–‘―ßœΑ–ΓΉι…ηΦΤΝΥ»γœ¬ΖΫΑΗ÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷ Θ§Ρψ»œΈΣΖΫΑΗΩ…––ΒΡ «__(Χν–ρΚ≈)
ΔΌ≈δ÷Τ“ΜΕ®ΝΩΒΡ0.10mol/LCH3COOH»ή“ΚΘ§»ΜΚσ≤β»ή“ΚΒΡpHΘ§»τpH¥σ”Ύ1Θ§‘ρ÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷
ΔΎ”Ο¥ΉΥα»ή“ΚΚΆ―ΈΥαΉωΒΦΒγ–‘ Β―ιΘ§»τ¥ΉΥα»ή“ΚΒΦΒγ–‘»θΘ§‘ρ÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷
ΔέΫΪpH=2ΒΡCH3COOH»ή“ΚΦ”Υ°œΓ Ά100±ΕΚσΘ§»τpH>4Θ§‘ρ÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷
Θ®2Θ©»τ25Γφ ±Θ§0.10mol/LΒΡCH3COOHΒΡΒγάκΕ»ΈΣ1%Θ§‘ρΗΟ»ή“ΚΒΡpH=___Θ§”…¥ΉΥαΒγάκ≥ωΒΡc(H+)‘ΦΈΣΥ°Βγάκ≥ωΒΡc(H+)ΒΡ___±ΕΓΘ
ΓΨ¥πΑΗΓΩΔΌ 3 108
ΓΨΫβΈωΓΩ
Θ®1Θ©“Σ÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷ Θ§÷Μ“Σ÷ΛΟς¥ΉΥα≤ΩΖ÷ΒγάκΦ¥Ω…ΘΜ
Θ®2Θ©ΗυΨίΒγάκΕ»ΒΡΗ≈ΡνΦΑpHΒΡ±μ¥ο ΫΦΤΥψ«βάκΉ”≈®Ε»Θ§ΗυΨίΥ°ΒΡάκΉ”ΜΐΦΤΥψΥ°Βγάκ≥ω«βάκΉ”≈®Ε»ΓΘ
(1)ΔΌ≈δ÷Τ“ΜΕ®ΝΩΒΡ0.10mol/LCH3COOH»ή“ΚΘ§»ΜΚσ≤β»ή“ΚΒΡpHΘ§»τpH¥σ”Ύ1Θ§ΥΒΟς»ή“Κ÷–c(H+)<c(CH3COOH)Θ§‘ρ¥ΉΥα≤ΩΖ÷ΒγάκΘ§Υυ“‘÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷ Θ§Ι ’ΐ»ΖΘΜ
ΔΎ»ή“ΚΒΦΒγ–‘”κάκΉ”≈®Ε»≥…’ΐ±»Θ§”Ο¥ΉΥα»ή“ΚΚΆ―ΈΥαΉωΒΦΒγ–‘ Β―ιΘ§»τ¥ΉΥα»ή“ΚΒΦΒγ–‘»θΘ§ΥΒΟς¥ΉΥα»ή“Κ÷–άκΉ”≈®Ε»–ΓΘ§ΒΪ «ΝΫ÷÷»ή“ΚΒΡ≈®Ε» «ΖώœύΒ»Έ¥÷ΣΘ§Υυ“‘≤ΜΡή÷ΛΟς¥ΉΥαΈΣ»θΒγΫβ÷ Θ§Ι ¥μΈσΘΜ
ΔέΫΪpH=2ΒΡCH3COOH»ή“ΚΦ”Υ°œΓ Ά100±ΕΚσΘ§»γΙϊ¥ΉΥαΦΧ–χΒγάκΘ§‘ρ»ή“ΚΒΡpH”ΠΗΟ–Γ”Ύ4Θ§Ι ¥μΈσΘ§Ι ¥πΑΗΈΣΘΚΔΌΘΜ
(2)»τ25Γφ ±Θ§0.10mol/LΒΡCH3COOHΒΡΒγάκΕ»ΈΣ1%Θ§»ή“Κ÷–c(H+)=cΓΝΠΝ=0.10mol/LΓΝ1%=0.001mol/LΘ§‘ρΗΟ»ή“Κ÷–pH=lgc(H+)=lg0.001=3ΘΜΗΟ»ή“Κ÷–Υ°Βγάκ≥ωΒΡc(H+)=![]() =1011mol/LΘ§”…¥ΉΥαΒγάκ≥ωΒΡc(H+)‘ΦΈΣΥ°Βγάκ≥ωΒΡc(H+)ΒΡ±Ε ΐ=
=1011mol/LΘ§”…¥ΉΥαΒγάκ≥ωΒΡc(H+)‘ΦΈΣΥ°Βγάκ≥ωΒΡc(H+)ΒΡ±Ε ΐ=![]() =108Θ§Ι ¥πΑΗΈΣΘΚ3ΘΜ108ΓΘ
=108Θ§Ι ¥πΑΗΈΣΘΚ3ΘΜ108ΓΘ