��Ŀ����
10��A��B��C��D�����ֳ����ĵ��ʣ�A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ�����֮���ת����ϵ��ͼ��ʾ��
��ش��������⣺
��1��B���Ӧ�Ļ�ѧ����ʽ��3Fe3O4+8Al$\frac{\underline{\;����\;}}{\;}$9Fe+4Al2O3��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����У��Ƿ��ܽ⣿����ǡ�����
��3����������ˮ�����Һ��������������ӵķ�����ȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+
��4��д��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
��5����A��B���ֽ�����һ������������ɻ���
��ȡһ�������ĸû��������м���������NaOH��Һ���������������ڱ�״����Ϊn L��B��NaOH��Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2�����������B�����ʵ���Ϊ$\frac{n}{33.6}$mol���ú���ĸ�ķ���ʽ��ʾ����
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����ΪmL���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ$\frac{m}{11.2}$mol���������A������Ϊ$\frac{m-n}{22.4}$g���ú���ĸ�ķ���ʽ��ʾ����
���� A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ����ת����ϵ��֪����Ϊ������������BΪAl��AΪFe��CΪ��������Ϊƫ�����ƣ���Ϊ�Ȼ�����Ȼ����Ԫ�ػ�����֪ʶ����ѧ���������
��� �⣺A��BΪ������C��D�����������壬��DΪ����ɫ���壮�ס��ҡ���Ϊ�����Ļ�������Ǻ�ɫ�Ҿ��д��Ե����ʣ����ת����ϵ��֪����Ϊ������������BΪAl��AΪFe��CΪ��������Ϊƫ�����ƣ���Ϊ�Ȼ�����
��1��B���Ӧ�Ļ�ѧ����ʽΪ3Fe3O4+8Al$\frac{\underline{\;����\;}}{\;}$9Fe+4Al2O3��
�ʴ�Ϊ��3Fe3O4+8Al$\frac{\underline{\;����\;}}{\;}$9Fe+4Al2O3��
��2�������£���A��B�ĵ��ʷ���Ũ�����Ũ�����з����ۻ���������ȫ�ܽ⣬
�ʴ�Ϊ����
��3�����������ӵķ���Ϊȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+��
�ʴ�Ϊ��ȡ����������Һ���Թ��У��μ�KSCN��Һ������Һ��죬˵�����д���Fe3+��
��4��A��ˮ������Ӧ����C�ͼĻ�ѧ����ʽΪ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
�ʴ�Ϊ��3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
��5����B��NaOH��Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO2-+3H2�����������������ڱ�״����Ϊn L��n��H2��=$\frac{n}{22.4}$mol����n��Al��=$\frac{n}{22.4}$mol��$\frac{2}{3}$=$\frac{n}{33.6}$mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����$\frac{n}{33.6}$��
����ȡ��ͬ�����ĸû��������м���������ϡ���ᣬ����ȫ���ܽ⣬�������������ڱ�״����Ϊm L��ת�Ƶ���Ϊ$\frac{m}{22.4}$��2����1-0��=$\frac{m}{11.2}$mol���������A������Ϊx���ɵ����غ��֪��$\frac{n}{33.6}$mol��3+$\frac{x}{56}$mol��2=$\frac{m}{22.4}$��2����1-0�������x=$\frac{m-n}{22.4}$��
�ʴ�Ϊ��$\frac{m}{11.2}$��$\frac{m-n}{22.4}$��
���� ���⿼��������ƶϣ�ע�����ȷ�Ӧ��DΪ����ɫ����Ϊ������ͻ�ƿڣ����ʵ��ƶ��ǽ����Ĺؼ��������еļ�����Ҫ�漰�����غ㼰��ϵʽ����Ŀ�Ѷ��еȣ�

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д���2��pHֵ��ͬ�� HCl��aq����H2SO4��aq����CH3COOH��aq����100mL
��������Һ�����ʵ���Ũ��������CH3COOH��
�ڷֱ���0.1mol/L��NaOH��aq���кͣ�����NaOH��aq��������ֱ�ΪV1��V2��V3�������ɴ�С��˳����V3��V1=V2��
�۷�Ӧ��ʼʱ����Ӧ����D������A��HCl��죻B��H2SO4��죻C��CH3COOH��죻D��һ���죩
��3���±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
| �¶�/�� | 25 | t1 | t2 |
| ˮ�����ӻ����� | 1��10-14 | �� | 1��10-12 |
����25��t1��t2�������1��10-14���������������=����
��25���£�ijNa2SO4��Һ��c��SO42-��=5��10-4 mol•L-1��ȡ����Һ1mL����ˮϡ����10mL����ϡ�ͺ���Һ��c ��Na+����c ��OH-��=1000��1
��t2���£���pH=11�Ŀ�������ҺV1 L��pH=1��ϡ����V2 L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1��V2=9��11������Һ�и������ӵ�Ũ���ɴ�С������˳����c��Na+����c��SO42-����c��H+����c��OH-��
��4��25��ʱ�������ΪVa��pH=a��ijһԪǿ�������ΪVb��pH=b��ijһԪǿ���ϣ�ǡ���кͣ�����֪Va��Vb��a=0.5b����a��ȡֵ��Χ��$\frac{7}{2}$��a��$\frac{14}{3}$��
| A�� | CO2���ӵĽṹʽ��O=C=O | |
| B�� | S2-�Ľṹʾ��ͼ�� | |
| C�� | R2+���Ӻ�����a�����ӣ�b�����ӣ�Rԭ�ӷ���Ϊ��${\;}_{a+2}^{a+b+2}$R | |
| D�� | ������ĵ���ʽ��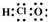 |
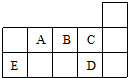
| A�� | ED4�����и�ԭ�Ӿ���8���ӽṹ | |
| B�� | AD3��ED4�����ӵ�����ԭ�Ӿ�Ϊsp3�ӻ� | |
| C�� | A��B��C��һ�����ܵĴ�С˳��ΪC��B��A | |
| D�� | C��D��̬�⻯���ȶ���ǿ���ͷе�ߵ;�ΪC��D |
| A�� | 1mol���к�������ĿΪ7NA | |
| B�� | ��״���£�22.4L�����й��ۼ���ĿΪ19NA | |
| C�� | 14g��ϩ�Ͷ�ϩ�Ļ�����к��е�ԭ������Ϊ3NA�� | |
| D�� | 1mol����ϩ��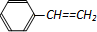 ���к��е�C=C��Ϊ4NA ���к��е�C=C��Ϊ4NA |
| A�� | Ư�۵ijɷ�Ϊ������ƣ��Ǵ����� | |
| B�� | ʵ���ҿ���Ũ������ﰱ�� | |
| C�� | ʵ���ҿ���NaOH��Һ����SO2��HCl���� | |
| D�� | ����Cl2��ʹ������ɫ������ɫ |
| A�� | �Ҵ��е�ˮ��CaO������ | B�� | �������е��Ҵ���ˮ����Һ�� | ||
| C�� | ���еļױ���Br2ˮ����Һ�� | D�� | �屽�е��壨NaOH��Һ����Һ�� |
 �����������н�����ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£�
�����������н�����ʹ���ã��ǽ���ʹ�õĽ�����ʹҩ���С����ȱ���֮�ƣ����Ʊ�ԭ�����£� NH2+CH3COOH$\stackrel{��}{?}$
NH2+CH3COOH$\stackrel{��}{?}$ NHCOOCH3+HO
NHCOOCH3+HO��֪��
�ٱ����ױ�������
�����������������ʹ���IJ��������������±���
| ���� | �۵� | �е� | �ܽ�� |
| �������� | 114.3�� | 305�� | ������ˮ����������ˮ |
| ���� | -6�� | 184.4�� | ����ˮ |
| ���� | 16.6�� | 118�� | ������ˮ |
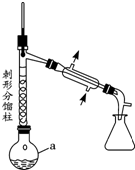
����1����a�У�����9mL ��0.10mol��������15mL��0.27mol�������ἰ����п�ۣ�������ͼװ����װ������
����2�������¶ȼƶ�����105�����ң�С����Ȼ�������Ӧ��ȫ��
����3�����Ƚ���Ӧ����ﵹ��ʢ��100mL ��ˮ���ձ��У���ȴ����ˣ�һ�ֿ��ٹ��˷�������ϴ�ӣ��õ��ֲ�Ʒ��
����4��������3���ôֲ�Ʒ��һ���ᴿ�Ƶò�Ʒ����Ϊ10.8g��
��ش��������⣺
��1������a������ΪԲ����ƿ����ѡ����a����ѹ����B������ţ���
A.25mL B.50mL C.100mL D.250mL
��2��ʵ���м�������п�۵�Ŀ���Ƿ�ֹ������������ͬʱ���ŷ�ʯ�����ã�
��3������2�У������¶ȼƶ�����105�����ҵ�ԭ�����¶ȹ��Ͳ���������Ӧ�����ɵ�ˮ���¶ȹ���δ��Ӧ������������
��4���жϷ�Ӧ�ѻ�����ȫ�ķ���Ϊ��ƿ������ˮ���ӣ�
��5������3�г��Ƚ�����ﵹ��ʢ����ˮ���ձ��У������ȡ���ԭ������ȴ���������ճ��ƿ���ϲ��״�����
��6������4�дֲ�Ʒ��һ���ᴿ�����ᴿ�������ؽᾧ��
��7������ʵ��IJ���Ϊ80%��
 ��
��