��Ŀ����
�����й���Һ������Ũ�ȵĹ�ϵʽ�У���ȷ����

A��pH��ͬ�Ģ�CH3COONa����NaHCO3����  ������Һ�е�c(Na��)���ۣ��ڣ���
������Һ�е�c(Na��)���ۣ��ڣ���
B��0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У� c(Na+)=2c(A2-)��c(HA-)��c(H2A)
C��ͼ��pH��7ʱ��c(Na��)��c(CH3COO��) ��c(OH��)��c(H��)
D��ͼ��a����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c(OH��)��c(H��)��c(CH3COO��)��2c(CH3COOH)
D
��������
���������A������Խ��Խˮ��Ĺ��ɣ�������ǿ������˳��Ϊ��CH3COOH>H2CO3>C6H5OH����ˮ��̶���ǿ����Ϊ��C6H5ONa>NaHCO3>CH3COONa����pH��ͬ��������Һ��c(Na+)Ϊ����>��>�ۣ�����B�����������غ�ɵ�0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У� c(Na+)=c(A2-)��c(HA-)��c(H2A)������C�����ݵ���غ�pH=7ʱ��c(Na��)=c(CH3COO��) ��c(OH��)��c(H��)������D�����ݵ���غ�ɵã�c(OH‾)+c(CH3COO‾)=c(Na+)+c(H+)�����������غ�ɵã�c(Na+)=2c(CH3COO‾)+2c(CH3COOH)�������غ��ϵʽ���ɵã�c(OH��)��c(H��)��c(CH3COO��)��2c(CH3COOH)����ȷ��
���㣺���⿼������ˮ����ɡ�����غ㡢�����غ㼰Ӧ�á�


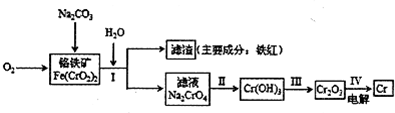
 Cr2O72-+H2O
Cr2O72-+H2O



