��Ŀ����
��2011?��̨ģ�⣩������ʴ�Ըߣ��ڸ��к����ﵽ12%��Ϊ����֣���ҵ��ұ��������Ҫ������ͼ��ʾ��
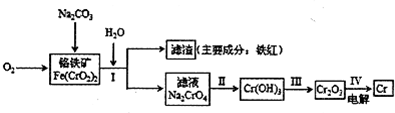
�Իش���������
��1�����粽���ķ�Ӧ����ʽΪ��
8Na2CrO4+6Na2S+23H2O��8Cr��OH��3��ʮ3Na2S2O3ʮ22NaOH
�÷�Ӧ����������
��2������IV�е��Cr2O3��������ӦʽΪ
��3�������£�������������ϡ���ᡢϡ���ᣬ��������Ũ���ᣮ�䲻����Ũ�����ԭ�������
��4�����磺2CrO42-+2H+ Cr2O72-+H2O
Cr2O72-+H2O
��25��C��KSP��Ag2Cr2O4��=1.12��10-12��KSP��Ag2Cr2O7��=2��10-7����Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ��
�ڽ�wg����Na2CrO4��������ˮ���lL��Һ������Һ���й����ӵ�Ũ�ȣ�mol?L-1����pH�Ĺ�ϵ���±���

���ϱ���֪��
��Ҫʹ��Һ��CrO42-�����ﵽ���Ӧ������Һ��pH
�� w g����Na2CrO4�����ʵ���n=

�Իش���������
��1�����粽���ķ�Ӧ����ʽΪ��
8Na2CrO4+6Na2S+23H2O��8Cr��OH��3��ʮ3Na2S2O3ʮ22NaOH
�÷�Ӧ����������
Na2CrO4
Na2CrO4
���ѧʽ�������� lmolCr��OH��3ʱת�Ƶ��ӵ����ʵ���Ϊ3
3
mol����2������IV�е��Cr2O3��������ӦʽΪ
2O2--4e-�TO2��
2O2--4e-�TO2��
����ʱCr2O3?��״̬ΪҺ̬��������̬��
Һ̬��������̬��
����3�������£�������������ϡ���ᡢϡ���ᣬ��������Ũ���ᣮ�䲻����Ũ�����ԭ�������
����������������Ĥ�������ۻ���
����������������Ĥ�������ۻ���
����4�����磺2CrO42-+2H+
 Cr2O72-+H2O
Cr2O72-+H2O��25��C��KSP��Ag2Cr2O4��=1.12��10-12��KSP��Ag2Cr2O7��=2��10-7����Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ��
Ag2CrO4
Ag2CrO4
���ڽ�wg����Na2CrO4��������ˮ���lL��Һ������Һ���й����ӵ�Ũ�ȣ�mol?L-1����pH�Ĺ�ϵ���±���

���ϱ���֪��
��Ҫʹ��Һ��CrO42-�����ﵽ���Ӧ������Һ��pH
��9
��9
���ã�������=��������ݱ�ʾ������ w g����Na2CrO4�����ʵ���n=
n��CrO42-��+c��Cr2O72-��+n��HCrO4-��
n��CrO42-��+c��Cr2O72-��+n��HCrO4-��
������ѧ����ʽ��ʾ������������1��������ϼۣ�ͨ�����ϼ۵ı仯�ж���������ת�Ƶ�����Ŀ��
��2��Cr2O3Ϊ���ӻ�������岻���磬����״̬�µ����Cr3+��O2-��O2-�������ŵ磻
��3��Ũ�������ǿ�����ԣ���ʹ���ۻ���
��4�������ܶȻ�������С���ж����ɳ��������ʣ�
��5���۲�������ݱ仯���ɣ������Ӧ��գ�
��2��Cr2O3Ϊ���ӻ�������岻���磬����״̬�µ����Cr3+��O2-��O2-�������ŵ磻
��3��Ũ�������ǿ�����ԣ���ʹ���ۻ���
��4�������ܶȻ�������С���ж����ɳ��������ʣ�
��5���۲�������ݱ仯���ɣ������Ӧ��գ�
����⣺��1��Cr���ϼ۴�+6��+3�����ϼ۽���3����Na2CrO4�������������� lmolCr��OH��3ʱת�Ƶ��ӵ����ʵ���Ϊ 3mol��
�ʴ�Ϊ��Na2CrO4 3��
��2������״̬ʱ��Cr2O3?�������룺Cr2O3�T2Cr3++3O2-�����Cr2O3��������ӦʽΪ��2O2--4e-�TO2����
�ʴ�Ϊ��2O2--4e-�TO2�� Һ̬��������̬����
��3��������������ϡ���ᡢϡ���ᣬ��������Ũ���ᣬ˵������Ũ�������������ܵı���Ĥ��
�ʴ�Ϊ������������������Ĥ�������ۻ�����
��4������KSP��Ag2Cr2O4��=1.12��10-12��KSP��Ag2Cr2O7��=2��10-7�������ܽ�ƽ�����ܽ��С�����С�ķ���ת����������Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó���Ϊ��Ag2CrO4��
�ʴ�Ϊ��Ag2CrO4��
�ڹ۲�������ݿ�֪��PH����c��CrO42-������PH=9ʱ��c��CrO42-��=0.996����Ҫʹ��Һ��CrO42-�����ﵽ���Ӧ������Һ��pH��9��
��Ԫ�ش�����ʽ�У�CrO42-��Cr2O72-��HCrO4-�����������غ�w g����Na2CrO4�����ʵ���Ϊ��n�Tn��CrO42-��+c��Cr2O72-��+n��HCrO4-����
�ʴ�Ϊ����9��n��CrO42-��+c��Cr2O72-��+n��HCrO4-����
�ʴ�Ϊ��Na2CrO4 3��
��2������״̬ʱ��Cr2O3?�������룺Cr2O3�T2Cr3++3O2-�����Cr2O3��������ӦʽΪ��2O2--4e-�TO2����
�ʴ�Ϊ��2O2--4e-�TO2�� Һ̬��������̬����
��3��������������ϡ���ᡢϡ���ᣬ��������Ũ���ᣬ˵������Ũ�������������ܵı���Ĥ��
�ʴ�Ϊ������������������Ĥ�������ۻ�����
��4������KSP��Ag2Cr2O4��=1.12��10-12��KSP��Ag2Cr2O7��=2��10-7�������ܽ�ƽ�����ܽ��С�����С�ķ���ת����������Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó���Ϊ��Ag2CrO4��
�ʴ�Ϊ��Ag2CrO4��
�ڹ۲�������ݿ�֪��PH����c��CrO42-������PH=9ʱ��c��CrO42-��=0.996����Ҫʹ��Һ��CrO42-�����ﵽ���Ӧ������Һ��pH��9��
��Ԫ�ش�����ʽ�У�CrO42-��Cr2O72-��HCrO4-�����������غ�w g����Na2CrO4�����ʵ���Ϊ��n�Tn��CrO42-��+c��Cr2O72-��+n��HCrO4-����
�ʴ�Ϊ����9��n��CrO42-��+c��Cr2O72-��+n��HCrO4-����
����������������ʵ�����dz����ܽ�ƽ����ƶ�����ͬ���͵��οɸ����ܶȻ��Ĵ�С���ж����������ɵ����ף�

��ϰ��ϵ�д�
�����Ŀ
 ��2011?��̨ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��
��2011?��̨ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��