��Ŀ����
(14��)
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PCl5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
��1����Ӧ�� �ܼ� SiO2+4HF=SiF4��+2H2O NaHCO3
��2��PF5+4H2O=H3PO4+5HF
��3������ ����
��4��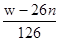
�����Ծ���������1������+Һ�������µ����ʼ�������Һ�����Ƿ�Ӧ����ܼ������ã���������Ҫ�ɷ�Ϊ�����κͶ������裬����HF��Ӧ��HF���������ԣ�������������к͡�
��2��PF5��PΪ+5�ۣ�FΪ-1�ۣ���ˮ��ΪH3PO4��HF��
��3����Һ�ķ�����ù��˵ķ�����HF��HCl�ķ��룬������е㲻һ����HF�д�������������з��룬�ʽ����¶ȣ�HF�ȱ�ΪҺ�壻
��4�������غ���Եõ�����LiPF6Ϊxmol��LiFΪymol������Li�غ㣬��x+y=n������������152x+26y=w�����Խ�á�
���㣺��ѧ���գ����ʵ����ʣ��������ᴿ����ѧ����

 ������������ϵ�д�
������������ϵ�д�������Ʒ��������Ӧ����
| A | B | C | D | ||
 ���� | 
|  ����� |  ������ | ||
| ���ǽ������� | �л��߷��Ӳ��� | �������� | ���ϲ��� |
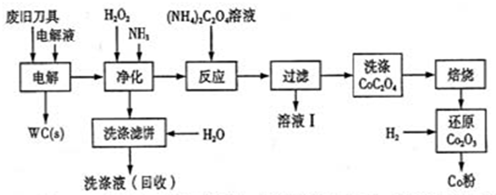
��ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
��֪�Ȼ��ƾ���Ļ�ѧʽ�ǣ�CaCl2��6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
��1����Ӧ���м������Ӧѡ��___________________��
��2����ɫ����Ӧ���������X��_______________���豸A��������______________���豸B������Ϊ________________���豸C��������____________________��
��3��Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����_____________����Ӧ�Ļ�ѧ����ʽΪ_________________��
| A��ˮ | B��Ũ���� | C��ʯ���� | D������ |
��5���ȼҵ�缫����ʽ_____________________��
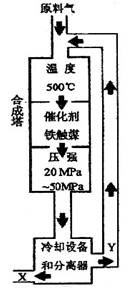
 2NH3+92.4KJ���ڻ�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ��ʾ��
2NH3+92.4KJ���ڻ�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ��ʾ��