��Ŀ����
13��ʵ����������80mL��1.5mol/L��NaHCO3��Һ���Իش���1����ʵ�����ʹ�õIJ��������ձ�����������100mL����ƿ����ͷ�ιܣ�
��2�����ø���ҺʱӦ��ȡNaHCO3������Ϊ12.6g��
��3�����в���������������Һ���ʵ���Ũ�ȵ�Ӱ�죨��д��Ӱ�졢ƫ��ƫ�ͣ�
A�����ƹ�����δϴ���ձ��Ͳ�������ƫ��
B������ƿʹ��֮ǰδ��ɣ�����������ˮ����Ӱ��
C������ʱ��������ƿ�Ŀ̶��ߣ�ƫ��
D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦����ƫ��
��4��д�����з�Ӧ�����ӷ���ʽ��
A����NaHCO3��Һ�еμ�����HCO3-+H+=CO2��+H2O
B����Ba��OH��2��Һ�еμ�����NaHCO3��ҺHCO3-+Ba2++OH-=H2O+BaCO3����
C����ˮ�еμ�MgCl2��Һ2NH3•H2O+Mg2+=Mg��OH��2��+2NH4+��
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2����������m=CVM�����㣻
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��4��A��̼�����ƺ�ϡ���ᷴӦ����NaCl��������̼��ˮ��
B����Ba��OH��2��Һ�еμ�����NaHCO3��Һ�������ٵ�NaHCO3Ϊ1mol����������Ҫ��ȷ�����ĵ��������������ӵ�����
C��NH3•H2O��������ʣ����ܲ�
��� �⣺��1������ʵ������80mL����ƿ����Ӧѡ��100mL����ƿ���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��Ҫ�������У�������ƽ���ձ�����������100mL����ƿ����ͷ�ιܣ����в��������У��ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ���ձ�����������100mL����ƿ����ͷ�ιܣ�
��2������ʵ������80mL����ƿ����Ӧѡ��100mL����ƿ�����Ƴ�100mL����Һ��������NaHCO3������m=CVM=1.5mol/L��0.1L��84g/mol=12.6g���ʴ�Ϊ��12.6g��
��3��A�����ƹ�����δϴ���ձ��Ͳ��������ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ��
B��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʴ�Ϊ����Ӱ�죻
C������ʱ��������ƿ�Ŀ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
D����������Һ������ƿת�Ƶ��Լ�ƿʱ��������Һ�彦�����ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��4��A��̼�����ƺ�ϡ���ᷴӦ����NaCl��������̼��ˮ�����ӷ���ʽΪHCO3-+H+=CO2��+H2O���ʴ�Ϊ��HCO3-+H+=CO2��+H2O��
B�������ٵ�NaHCO3Ϊ1mol����1molHCO3-������1molOH-��1molBa2+����HCO3-+Ba2++OH-=H2O+BaCO3�����ʴ�Ϊ��HCO3-+Ba2++OH-=H2O+BaCO3����
C��NH3•H2O��������ʣ����ܲ𣬹���ˮ�еμ�MgCl2��Һ�����ӷ���ʽΪ��
2NH3•H2O+Mg2+=Mg��OH��2��+2NH4+���ʴ�Ϊ��2NH3•H2O+Mg2+=Mg��OH��2��+2NH4+��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ��㡢���������Լ����ӷ���ʽ����д�����ڻ�������Ŀ���ѶȲ���

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| ������ʽ | C16H14O2 |
| �������� | ��ʹBr2/CCl4��ɫ |
| ����ϡH2SO4��ˮ�� |
��2���ɼ�����ȡ�߾���K����д���÷�Ӧ�ķ���ʽ
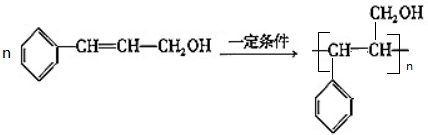
��3���ɼ�ת��Ϊ���辭���й��̣�����ȥ������Ӧ���ز����ͬ����
��$��_{��}^{һ������}$
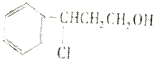 $��_{��}^{O_{2}/Cu}$Y$��_{��}^{һ������}$��
$��_{��}^{O_{2}/Cu}$Y$��_{��}^{һ������}$�����з�Ӧ��ķ�Ӧ����Ϊ�ӳɷ�Ӧ����Ӧ��Ļ�ѧ����ʽΪ
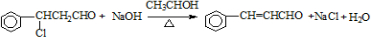 ��ע����Ӧ��������
��ע����Ӧ����������4����֪��RCH=CHR��$��_{ii��Zn/H_{2}O}^{i��O_{3}}$RCHO+R��CHO��2HCHO$��_{ii��H+}^{i��ŨNaOH}$HCOOH+CH3OH
�����Ʊ���һ�ֺϳ�·��ͼ��ͼ2��A��F��Ϊ�л��ͼ��Mr��ʾ��Է�����������
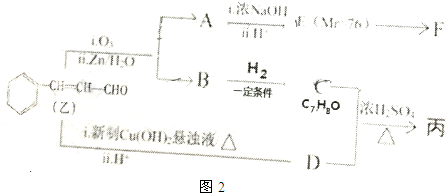
��C��D����������Ӧ���ɱ�������Ľṹ��ʽΪ
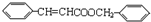 ��
�����������ʲ�����C��Ӧ����bc��ѡ����ţ�
a�������� b���������� c��Na2CO3��Һ d������
��F����������E���ɵ���Ԫ����д��F�Ľṹ��ʽ
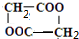 ��
����ͬʱ��������������D��ͬ���칹����10�֣�
a��������������ȡ���� b���ܷ���������Ӧ c������Br2��CCl4��Һ�����ӳɷ�Ӧ d�����ڷ���
д����������һ�ֵĽṹ��ʽ
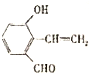 ��
�� | A�� | ��������� | B�� | O2��O3 | C�� | 12C��14C | D�� | ��������춡�� |

| A�� | CH3OCH3 | B�� | CH3CH2OH | C�� | CH3COOH | D�� | CH3OH |
| A�� | Ħ������g/mol | B�� | ����Ħ�����L/mol | ||
| C�� | ���ʵ����ĵ�λKg | D�� | �ܶ�g/cm3 |

 ijͬѧ����֪ȷŨ�ȵĸ��������Һ�ζ���Һ��Fe2+��Ũ�ȣ����������ҺӦʢ���ڼ��У���ס����ҡ���
ijͬѧ����֪ȷŨ�ȵĸ��������Һ�ζ���Һ��Fe2+��Ũ�ȣ����������ҺӦʢ���ڼ��У���ס����ҡ���