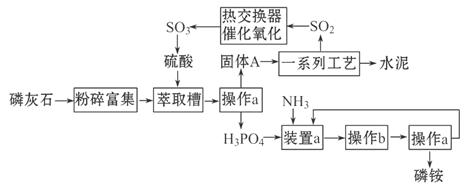
��Ŀ����
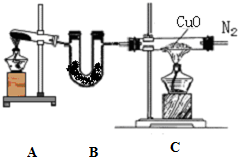
ijͬѧ����ʵ�����ü��ȹ���ķ�����ȡһƿ��������������Ȫʵ�飮����Ƶ�ʵ��װ����ͼ��ʾ��ͼ2�еļг�װ������ȥ����
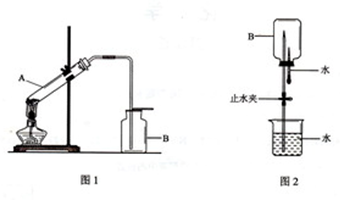
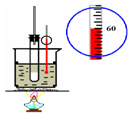
��1��ͼ1��ʾװ���еĴ���Ϊ______��
��2����ͼ1��ʾװ���еĴ�����������ʵ�飬�Թ�A����������Ӧ�Ļ�ѧ����ʽΪ______��һ��ʱ���һ��պ��Ũ����IJ������ӽ�����ƿ�ڣ������������̣��÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3�������ռ��������ļ���ƿB����ͼ2��ʾ���Ӻ�װ�ý�����Ȫʵ��ʱ������ˮ����IJ�����______
______����Ԥ����ˮ�еμ�������̪��Һ�����۲쵽______ɫ����Ȫ��
��4��Ũ��ˮ�����ڼ��������ܵ��Ƿ�©������ԭ����8NH3+3Cl2=6NH4Cl+N2���ڸ÷�Ӧ�У������ɱ�״����11.2LN2ʱ�������������ʵ�������______g��

��1��ͼ1��ʾװ���еĴ���Ϊ______��
��2����ͼ1��ʾװ���еĴ�����������ʵ�飬�Թ�A����������Ӧ�Ļ�ѧ����ʽΪ______��һ��ʱ���һ��պ��Ũ����IJ������ӽ�����ƿ�ڣ������������̣��÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3�������ռ��������ļ���ƿB����ͼ2��ʾ���Ӻ�װ�ý�����Ȫʵ��ʱ������ˮ����IJ�����______
______����Ԥ����ˮ�еμ�������̪��Һ�����۲쵽______ɫ����Ȫ��
��4��Ũ��ˮ�����ڼ��������ܵ��Ƿ�©������ԭ����8NH3+3Cl2=6NH4Cl+N2���ڸ÷�Ӧ�У������ɱ�״����11.2LN2ʱ�������������ʵ�������______g��
��1�����������Ҫ�Թܿ���������б����ֹ�г���ˮ�������Թܵײ�ը���Թܣ������DZȿ�����ļ�������ˮ�����壬��Ҫ�������������ռ���װ���еĴ����ǣ����Թܹܿ�������б������ƿ���ռ����������������ռ���
�ʴ�Ϊ�����Թܹܿ�������б������ƿ���ռ����������������ռ���
��2��ʵ��������ʯ�Һ��Ȼ���ڼ��������·�Ӧ�Ʊ���������Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��һ��ʱ���һ��պ��Ũ����IJ������ӽ�����ƿ�ڣ������������̣��÷�Ӧ�Ļ�ѧ����ʽΪ��NH3+HCl=NH4Cl��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��NH3+HCl=NH4Cl��
��3��������������ˮ�������ֹˮ�У���������ˮ����ƿ��ѹǿѸ�ټ�С�����γ���Ȫ��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ���������ܽ���ˮ����һˮ�ϰ��������Һ�д��ڵ���ƽ�⣬��������������������Լ��ԣ�������̪���ɫ�����Թ۲쵽��ɫ��Ȫ��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ���죻
��4��Ũ��ˮ�����ڼ��������ܵ��Ƿ�©������ԭ����8NH3+3Cl2=6NH4Cl+N2���ڸ÷�Ӧ�У������ɱ�״����11.2LN2ʱ�����ʵ���=
=0.5mol�����ݻ�ѧ����ʽ������Ԫ�ػ��ϼ۴�-3�۱仯Ϊ0�ۣ�������������ÿ����1molN2���������İ������ʵ���Ϊ2mol����������0.5molN2���������İ������ʵ���Ϊ1mol������=17g/mol��1mol=17g��
�ʴ�Ϊ��17��
�ʴ�Ϊ�����Թܹܿ�������б������ƿ���ռ����������������ռ���
��2��ʵ��������ʯ�Һ��Ȼ���ڼ��������·�Ӧ�Ʊ���������Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��3��������������ˮ�������ֹˮ�У���������ˮ����ƿ��ѹǿѸ�ټ�С�����γ���Ȫ��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ���������ܽ���ˮ����һˮ�ϰ��������Һ�д��ڵ���ƽ�⣬��������������������Լ��ԣ�������̪���ɫ�����Թ۲쵽��ɫ��Ȫ��
�ʴ�Ϊ����ֹˮ�У�������ͷ�ι��е�ˮ���죻
��4��Ũ��ˮ�����ڼ��������ܵ��Ƿ�©������ԭ����8NH3+3Cl2=6NH4Cl+N2���ڸ÷�Ӧ�У������ɱ�״����11.2LN2ʱ�����ʵ���=
| 11.2L |
| 22.4L/mol |
�ʴ�Ϊ��17��

��ϰ��ϵ�д�
 С�����ϵ�д�
С�����ϵ�д�
�����Ŀ