��Ŀ����
����Ŀ��Ϊ��ȥ�����е�Ca2����Mg2����Fe3����SO42���Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
![]()
![]()
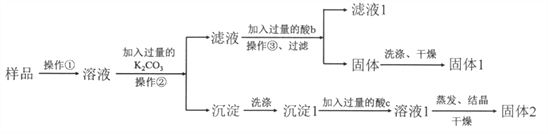
��1���ж�BaCl2�ѹ����ķ����� ��
��2���������У���صĻ�ѧ����ʽ�� ��
��3������NaCl��Һʱ�����������в���������ƫ����ƫ�ͣ�
A������ʱNaCl�ѳ��⣨ �� |
B����ƽ����������ʴ�� �� |
C������ҡ��ʱ��Һ���½��ּ�ˮ�� �� |
D������ʱ���ӿ̶��ߣ� �� |
���𰸡���1��ȡ����������ϲ���Һ1��2�Σ����ڵ�ΰ��ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ�����
��2��CaCl2��Na2CO3==CaCO3����2NaCl��BaCl2��Na2CO3==BaCO3����2NaCl
��3��A.ƫ�� B��ƫ�� C��ƫ�� D��ƫ��
�������������������1��BaCl2�ѹ��������ټ��Ȼ����������ɳ�������ʵ�鷽��Ϊ���Թ�ȡ������������������ϲ���Һ���ٵ��뼸��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ������ʴ�Ϊ�����Թ�ȡ������������������ϲ���Һ���ٵ��뼸��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ�����
��2�����ε��ᴿ�У�����̼���Ƶ������dz�ȥ�������Ӹ������Լ������ı����ӣ���Ӧ�Ļ�ѧ����ʽΪCaCl2+Na2CO3=CaCO3��+2NaCl��BaCl2+Na2CO3=BaCO3��+2NaCl���ʴ�Ϊ��CaCl2+Na2CO3=CaCO3��+2NaCl��BaCl2+Na2CO3=BaCO3��+2NaCl��
��3��A������ʱ NaCl �ѳ��⣬��ʵ�ʳ����Ȼ�������ƫС��������ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B����ƽ����������ʴ����ʵ�ʳ�����������ƫ������Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
C������ҡ��ʱ��Һ���½��ּ�ˮ��������Һ���ƫ��Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
D������ʱ���ӿ̶��ߣ�������Һ���ƫС��Ũ��ƫ�ʴ�Ϊ��ƫ�ߡ�

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�