��Ŀ����
8�������ȼ�ϵ����һ�����͵�أ�������ӵ�ؾ��и��ߵ������ܶȣ�����ʱҲ�����磬����ȼ�ϵ��ʱ���ɸ���������ˮ�Ե��Һ�Ϳ����Լ������Ľ���﮾Ϳ�������ʹ�ã��������������﮿ɲ��õ�������������﮶�ѭ��ʹ�ã��乤��ʾ��ͼ����ͼ������˵������ȷ���ǣ�������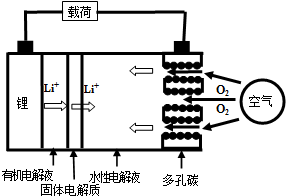
| A�� | �ŵ�ʱ�����ĵ缫��ӦʽΪ��Li-e-=Li+ | |
| B�� | ���ڵ���������ö��Ե缫�������ʱ��������������õ� | |
| C�� | �л����Һ�������Ҵ�����ˮ�л��� | |
| D�� | �ŵ�ʱ�������ĵ缫��ӦʽΪ��2H2O+O2+4e-=4OH- |
���� ��﮿�������У�����������Կ����е�������Ϊ������Ӧ�ˮ�Ե��Һ�������õ����������������ӣ��ݴ˽��
��� �⣺A�������Ϊ����������Ϊ�����������缫��ӦʽΪ��Li-e-=Li+����A��ȷ��
B�����ڵ���������ö��Ե缫�������ʱ������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��Li++e-=Li����B��ȷ��
C���������Ҵ�����Ϊ�Ҵ�������Ҫ�����û���Ӧ����C����
D������Ϊ������������ӦΪO2+2H2O+4e-=4OH-����D��ȷ��
��ѡC��
���� ���⿼��绯ѧ����֪ʶ���漰�缫�жϡ��缫����ʽ��д�����֪ʶ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
13��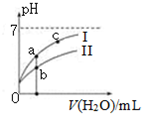 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��ͼ��ʾ�����£�ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ���ǣ�������
 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | ��ͬŨ�ȵ�CH3COONa��NaClO�Ļ����Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽΪ��ClO-+CO2+H2O=HClO+CO32- | |
| C�� | ͼ����a��c���㴦����Һ��$\frac{c��{R}^{-}��}{c��HR��•c��O{H}^{-}��}$��ȣ�HR����CH3COOH��HClO�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |
14������˵����ȷ���ǣ�������
| A�� | ��ʹ�����Ժ�ɫ����Һ�д������ڣ�Mg2+��Na+��Cl-��F- | |
| B�� | ��״���£�46gNO2��N2O4��������к���ԭ�Ӹ���Ϊ3NA | |
| C�� | 1L0.5mol•L-1 CuSO4��Һ�к���0.5NA��Cu2+ | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol/L�İ�ˮ�����ᡢ��ˮ�������c��H+���������ˮ |
11�����з���ʽ��ʾ���л����ͬ���칹�干�����ֵ��ǣ������������칹����������
| A�� | C3H7Cl | B�� | C3H6Cl2 | C�� | C3H5Cl3 | D�� | C3HCl7 |
3����������Ԫ�صĻ�̬ԭ�ӵĵ����Ų�ʽ���£���1s22s22p63s23p4����1s22s22p63s23p3����1s22s22p5����1s22s22p63s23p2�������йرȽ�����ȷ���ǣ�������
| A�� | ��һ�����ܣ��ۣ��ڣ��ܣ��� | B�� | ԭ�Ӱ뾶���٣��ڣ��ܣ��� | ||
| C�� | �縺�ԣ��ۣ��٣��ڣ��� | D�� | ��������ϼۣ��ۣ��٣��ڣ��� |
20�������濼�Ի�ѧ�Ծ�������ǰ���С������õ������ԭ��������һ���H-1��C-12��Cl-35.5��N-14�ȣ�������Щ����ȷ��˵��Ӧ���ǣ�������
| A�� | ij�ֺ��ص����ԭ������ | |
| B�� | ij�ֺ��ص������� | |
| C�� | ij��Ԫ�����к�����������ƽ��ֵ | |
| D�� | ij��Ԫ�ص�ƽ�����ԭ�������Ľ���ֵ |
17����11��17��Ԫ�����ʵ�������ȷ���ǣ�������
| A�� | ԭ�Ӱ뾶�ͼ����Ӱ뾶��С | |
| B�� | �������Ӧ��ˮ������Լ�����������ǿ | |
| C�� | 14��Ԫ�صĸߴ����ʿ��ƹ��� | |
| D�� | ���ʵ��۵㽵�� |
18���ں��д�����Na+��OH-��NO3-������Һ�л����ܴ������ڵ������ǣ�������
| A�� | NH4+ | B�� | H+ | C�� | SO42- | D�� | Mg2+ |

