��Ŀ����
����Ŀ������������(NOx)�Ǵ�����Ⱦ��֮һ����ҵ����һ���¶Ⱥʹ�����������NH3��NOx��ԭ����N2��ijͬѧ��ʵ�����ж�NH3��NOx��Ӧ������̽�����ش��������⣺
��1���������Ʊ�

�ٰ����ķ���װ�ÿ���ѡ����ͼ�е�_________����Ӧ�Ļ�ѧ����ʽΪ_______________��
�����ռ�һƿ����İ�����ѡ����ͼ�е�װ�ã�������˳��Ϊ������װ�á�______(������������Сд��ĸ��ʾ)��
��2����������������ķ�Ӧ
�������ռ�����NH3����ע����X�У�Ӳ�ʲ�����Y�м�����������������NO2(�����ü���K1��K2�к�)����һ���¶��°�ͼʾװ�ý���ʵ�顣
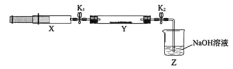
�������� | ʵ������ | ����ԭ�� |
��K1���ƶ�ע����������ʹX�е����建��ͨ��Y���� | ��Y����_____________ | �ڷ�Ӧ�Ļ�ѧ����ʽ ____________ |
��ע���������˻�ԭ�����̶�����װ�ûָ������� | Y����������ˮ�� | ���ɵ���̬ˮ���� |
��K2 | ��_______________ | ��______________ |
���𰸡���1���� A�� 2NH4Cl+Ca(OH)2![]() 2NH3��+ CaCl2+2H2O
2NH3��+ CaCl2+2H2O
(��B��NH3��H2O![]() NH3��+H2O)����d c f e i��
NH3��+H2O)����d c f e i��
��2��������ɫ����������dz����8NH3+6NO2 ![]() 7N2 +12H2O��
7N2 +12H2O��
��Z��NaOH��Һ�����������ܷ�Ӧ��������������٣�Y����ѹǿС����ѹ��
����������1������ʵ�����У���������������NH4Cl��ϼ��ȷ������ֽⷴӦ��ȡ����������������NH4Cl���ǹ��壬�������������ȡ���壬��Ӧѡ��Aװ�ý��У���ȡ������������Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��Ҳ��������NH3��H2O�IJ��ȶ��ԣ����������ȷֽ����������ˮ��ȡ��������ʱӦ��ѡ���װ����װ��B����Ӧ�Ļ�ѧ����ʽ�ǣ�NH3��H2O
CaCl2+2NH3��+2H2O��Ҳ��������NH3��H2O�IJ��ȶ��ԣ����������ȷֽ����������ˮ��ȡ��������ʱӦ��ѡ���װ����װ��B����Ӧ�Ļ�ѧ����ʽ�ǣ�NH3��H2O![]() NH3��+H2O��������A(��B)װ���Ƶõİ��������ڰ����Ǽ������壬������Ҫ���ü��Ը������ʯ�ҽ��и��Ȼ���ٸ��ݰ������ܶȱȿ���С�����ʣ��������ſ������ռ��������Ǵ�����Ⱦ�Ҫ����β������������������ˮ�м����ܽ�����ʣ���ˮ�����ռ�����β����������װ�õ�����˳��Ϊd��c��f��e��i����2����NO2����ǿ�����ԣ�NH3��ǿ��ԭ�ԣ������������ᷢ��������ԭ��Ӧ����������ˮ�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�ķ���ʽ��8NH3+6NO2
NH3��+H2O��������A(��B)װ���Ƶõİ��������ڰ����Ǽ������壬������Ҫ���ü��Ը������ʯ�ҽ��и��Ȼ���ٸ��ݰ������ܶȱȿ���С�����ʣ��������ſ������ռ��������Ǵ�����Ⱦ�Ҫ����β������������������ˮ�м����ܽ�����ʣ���ˮ�����ռ�����β����������װ�õ�����˳��Ϊd��c��f��e��i����2����NO2����ǿ�����ԣ�NH3��ǿ��ԭ�ԣ������������ᷢ��������ԭ��Ӧ����������ˮ�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�ķ���ʽ��8NH3+6NO2![]() 7N2 +12H2O�����ݷ�Ӧ����ʽ��֪��Ӧ�����ǣ�Y���ڿ�������ɫ����������dz��ͬʱ��ע�������ڱ���ˮ���������������������֪�����÷�Ӧ�ķ���ʽ��8NH3+6NO2
7N2 +12H2O�����ݷ�Ӧ����ʽ��֪��Ӧ�����ǣ�Y���ڿ�������ɫ����������dz��ͬʱ��ע�������ڱ���ˮ���������������������֪�����÷�Ӧ�ķ���ʽ��8NH3+6NO2![]() 7N2 +12H2O������Ӧ��������������������ʵ������٣����Ի�ʹ����������ѹǿ��С������K2���ձ���NaOH��Һ�ڴ���ѹǿ�������»ᵹ������Y���ڣ�����Һ����Y���ڵ�ԭ���������÷�Ӧ�����������С�ķ�Ӧ����Ӧ��������װ����ѹǿ���ͣ���������ѹ�������¶�������������
7N2 +12H2O������Ӧ��������������������ʵ������٣����Ի�ʹ����������ѹǿ��С������K2���ձ���NaOH��Һ�ڴ���ѹǿ�������»ᵹ������Y���ڣ�����Һ����Y���ڵ�ԭ���������÷�Ӧ�����������С�ķ�Ӧ����Ӧ��������װ����ѹǿ���ͣ���������ѹ�������¶�������������