��Ŀ����
ij�¶�(T��)ʱ�����0.01mol/LNaOH��Һ��pHΪ11������¶���ˮ��KW��____________������¶�________(����ڡ�����С�ڡ����ڡ�)���£���������_______________________________________________________________________��
���¶��£���pH��a��NaOH��ҺVaL��pH��b��H2SO4��ҺVbL��ϣ�ͨ��������д���²�ͬ���ʱ����Һ������ȣ�
(1)�����û����ҺΪ���ԣ���a��12��b��2����Va��Vb��________��
(2)�����û����ҺΪ���ԣ���a��b��12����Va��Vb��________��
(3)�����û����Һ��pH��10����a��12��b��2����Va��Vb��__________��
���¶��£���pH��a��NaOH��ҺVaL��pH��b��H2SO4��ҺVbL��ϣ�ͨ��������д���²�ͬ���ʱ����Һ������ȣ�
(1)�����û����ҺΪ���ԣ���a��12��b��2����Va��Vb��________��
(2)�����û����ҺΪ���ԣ���a��b��12����Va��Vb��________��
(3)�����û����Һ��pH��10����a��12��b��2����Va��Vb��__________��
1.0��10��13�����ڡ�ˮ�ĵ��������ȵģ�����ʱˮ�ĵ���ƽ�������ƶ���KW��������¶��µ�KW��25��ʱ�����Ը��¶ȴ���25��
(1)1��10��(2)10��1��(3)1��9
(1)1��10��(2)10��1��(3)1��9
0.01mol/LNaOH��Һ��c��OH����=0.01mol/L��pH=11����c��H+��=10��11mol/L��KW��c��OH������c��H+��=1.0��10��13
�����û����ҺΪ���ԣ���c��OH����= c��H+��������
10a��13��Va = 10��b��Vb ����a��b��������𰸡�
�����û����Һ��pH��10>13/2����Ϊ���ԣ���
 �ɵô𰸡�
�ɵô𰸡�
�����û����ҺΪ���ԣ���c��OH����= c��H+��������
10a��13��Va = 10��b��Vb ����a��b��������𰸡�
�����û����Һ��pH��10>13/2����Ϊ���ԣ���
 �ɵô𰸡�
�ɵô𰸡�
��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
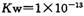 ������������ȷ����
������������ȷ����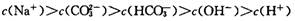
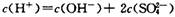
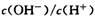 ��֮����
��֮���� ����
����