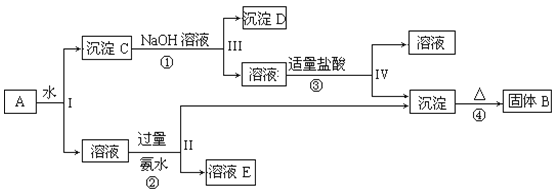
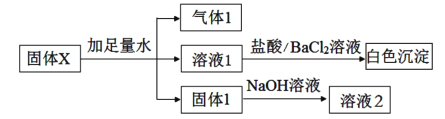
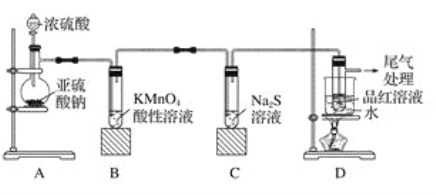
��Ŀ����
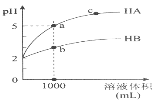
����Ŀ����֪Aת��ΪC��D�ֲ����У���A(g)![]() B(g)+2D(g)��B(g)
B(g)+2D(g)��B(g)![]() C(g)+D(g)���䷴Ӧ����������ͼ��ʾ������˵����ȷ���ǣ� ��
C(g)+D(g)���䷴Ӧ����������ͼ��ʾ������˵����ȷ���ǣ� ��
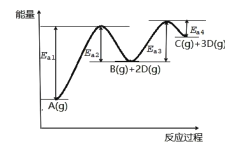
A.1molA(g)����������1molB(g)������
B.B(g)![]() C(g)+D(g) ��H=Ea4-Ea3
C(g)+D(g) ��H=Ea4-Ea3
C.����1molA(g)��ѧ�����յ�����С���γ�1molC(g)��3molD(g)��ѧ�����ų�������
D.��Ӧ�����У�����Ea3<Ea1����Ӧ�����ʴ��ڷ�Ӧ�٣�����B���Ѵ�������
���𰸡�D
��������
A. ��ͼ�п�֪1molA(g)����������1molB(g)��2molD(g)�������������ܱȽ�1molA(g)��������1molB(g)��������С��A����
B. ��ͼ�з�Ӧǰ�������仯��֪����Ӧ��������������������������B(g)![]() C(g)+D(g)Ϊ���ȷ�Ӧ����H>0������H=Ea3-Ea4��B����
C(g)+D(g)Ϊ���ȷ�Ӧ����H>0������H=Ea3-Ea4��B����
C. ��ͼ�п�֪��Aת��ΪC��DΪ���ȷ�Ӧ������1molA(g)��ѧ�����յ�����Ӧ�����γ�1molC(g)��3molD(g)��ѧ�����ų���������C����
D. �ӷ�Ӧ���̵�ͼ���п�֪�� Ea3<Ea1�����Խ�ͣ���Ӧ����Խ�죬�ʷ�Ӧ�����ʴ��ڷ�Ӧ�٣�����B���Ѵ������ۣ�D��ȷ��
��ѡD��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�����Ŀ�������Ǽ�����Ҫ�Ļ���ԭ�ϣ��ڹ�ҵ��ũҵ��ҽҩ�����µ�����Ӧ�ù㷺����ҵ��ͨ���ýӴ��������ᣬ��Ҫԭ����������Ϳ������Ӵ�����������������̴��¿ɷ�Ϊ�����Σ������������ȡ�;�������������ת��Ϊ��������������������պ���������ɡ�Ϊ�˷�ֹ������Ⱦ����β�������ۺ����ã����᳧���ð�ˮ����β����SO2��SO3�����壬��������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣Ϊ�˲ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ50.00mL��������120�����ң�ʹ����ȫ���ݳ�[��NH4��2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
ʵ�� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
��1����1������ֱ���Ʋ⣺1.81g��Ʒ����ͬ��ʵ��ʱ�����ɰ������������״����Ϊ___L��
��2���Լ���û�����У�NH4��2SO4�� NH4HSO4�����ʵ���֮��Ϊ___��
��3��������NaOH��Һ�����ʵ���Ũ��___mol/L��