��Ŀ����
��16�֣������������NOx��ʾ���Ǵ�����Ⱦ�������Դ�����
��1������β���е�CO��NOx���ô�ת����������ʹ�������Ӧ���ɲ������ѭ���������塣��Ӧ��ѧ����ʽ�ɱ�ʾΪ��_ _____________��
_____________��
��2����ҵβ���е��������ﳣ���ð������շ���ԭ����NH3��NOx��Ӧ�����������ʡ�ijͬѧ��������װ�ã�����ͼ���Ͳ���ģ�ҵ�ϵ��������ﴦ�����̡�
��.�ṩ��װ�á�
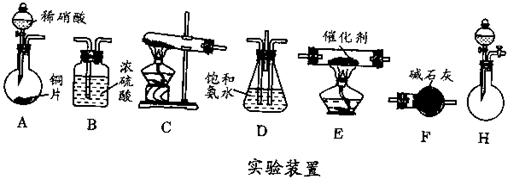
��.NH3����ȡ��
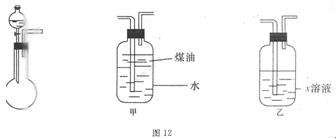
�����ṩ��װ�����ܿ��١������ȡNH3��װ���ǣ�__________����������ţ���
��������Cװ����ȡ����������ʵ��������ͬ������������ʾ��
ʵ���¼
�����������ݣ�ʵ������NH3������ߵ��ǣ�___________________������ţ����������NH3���ʲ��ߵ�ԭ���ǣ�____________��
��.ģ��β���Ĵ�����
ѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ã�
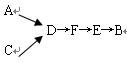
��A�з�Ӧ�����ӷ���ʽ��_________��
��Dװ�����ó�ʹ�����Ͼ��Ⱥ͵��������ٶ����⣬����һ��������_____________��
��Dװ���е�Һ��ɻ���_________������ţ���
a.CuSO4��Һ b.H2O c.CCl4 d.ŨH2SO4
�ܸ�ͬѧ����Ƶ�ģ��β������ʵ�黹���ڵ�����ȱ����____ ____________��
____________��
��1������β���е�CO��NOx���ô�ת����������ʹ�������Ӧ���ɲ������ѭ���������塣��Ӧ��ѧ����ʽ�ɱ�ʾΪ��_
 _____________��
_____________����2����ҵβ���е��������ﳣ���ð������շ���ԭ����NH3��NOx��Ӧ�����������ʡ�ijͬѧ��������װ�ã�����ͼ���Ͳ���ģ�ҵ�ϵ��������ﴦ�����̡�
��.�ṩ��װ�á�

��.NH3����ȡ��
�����ṩ��װ�����ܿ��١������ȡNH3��װ���ǣ�__________����������ţ���
��������Cװ����ȡ����������ʵ��������ͬ������������ʾ��
ʵ���¼
| �Լ������� | �����Լ�/g | NH3���/mL |
| a | 12.0g Ca(OH)2(����) 10.8g NH4Cl | 2688 |
| b | 12.0g Ca (OH)2(����) 10.8g(NH4)2SO4 (OH)2(����) 10.8g(NH4)2SO4 | 2728 |
| c | 12.0g NaOH(����) 10.8g NH4Cl | 3136 |
| d | 12.0g NaOH(����) 10.8g (NH4)2SO4 | 3118 |
| e | 12.0g CaO(����) 10.8g NH4Cl | 3506 |
| f | 12.0g CaO(����) 10.8g (NH4)2SO4 | 3584 |
��.ģ��β���Ĵ�����
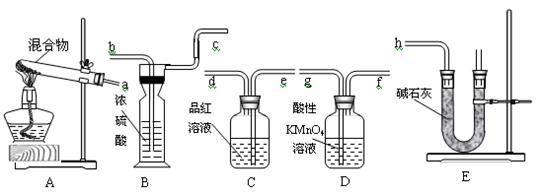
ѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ã�
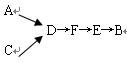
��A�з�Ӧ�����ӷ���ʽ��_________��
��Dװ�����ó�ʹ�����Ͼ��Ⱥ͵��������ٶ����⣬����һ��������_____________��
��Dװ���е�Һ��ɻ���_________������ţ���
a.CuSO4��Һ b.H2O c.CCl4 d.ŨH2SO4
�ܸ�ͬѧ����Ƶ�ģ��β������ʵ�黹���ڵ�����ȱ����____
 ____________��
____________����ÿ��2�֣���16�֣�


��

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
 ��
��
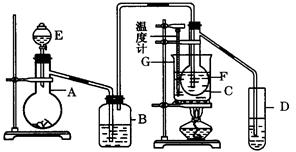
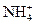

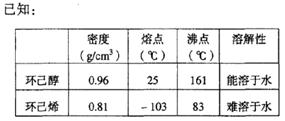
 �ڽ��롣����ʱҪ������ʯ�ң�Ŀ���� ��
�ڽ��롣����ʱҪ������ʯ�ң�Ŀ���� ��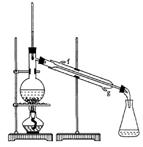
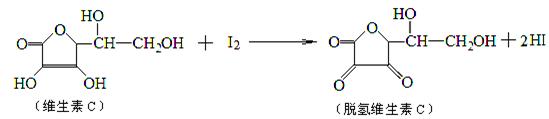
 ���ⶨij��֭��ά����C�ĺ������仯ѧ����ʽ������ʾ������˵����ȷ����
���ⶨij��֭��ά����C�ĺ������仯ѧ����ʽ������ʾ������˵����ȷ����