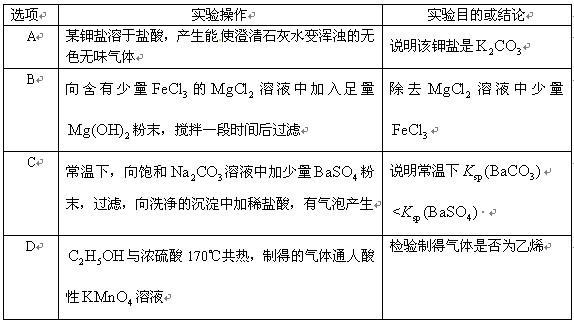
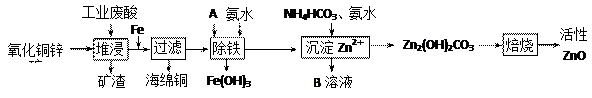��Ŀ����
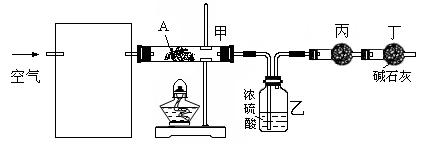
��75�����ң���HgSO4����������Ȳ��ˮ��Ϊ��ȩ����HgSO4����ijЩ�ض����ʳ��ᷢ�������ж���ʧȥ�����ã�H2S��������һ�֣�������Ȳˮ������ֻ�ÿ�״��ʯ��Ũ���ᡢˮ��NaOH��Һ��HgO��ĩ������������ȩ��װ����ͼ��
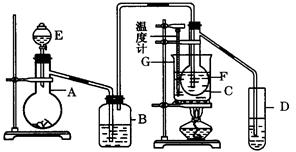
��ش��������⣺
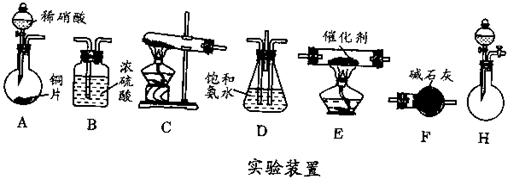
(1) ʵ�鿪ʼʱ������A��ʢ�ŵ�ʯ��B��Ӧװ�� ���������� ��
(2) ����D��ʢ��ˮ���������� ��
(3) ������ƿF��Ӧ����HgO�����������Լ����������߷ֱ�ֱ�Ӽ��룬�밴�������
��˳��д������HgO���ڵĸ��Լ����� ����HgO����F�еIJ��������� ��
(4) װ��ͼ�У�����F�ķ�ʽ�� ����ѡ���¶ȼ�G�����̱�ʾ��ȷ����__________������ţ���
(5) ������ȩ���Ƴ��IJ����������� ��

��ش��������⣺
(1) ʵ�鿪ʼʱ������A��ʢ�ŵ�ʯ��B��Ӧװ�� ���������� ��
(2) ����D��ʢ��ˮ���������� ��
(3) ������ƿF��Ӧ����HgO�����������Լ����������߷ֱ�ֱ�Ӽ��룬�밴�������
��˳��д������HgO���ڵĸ��Լ����� ����HgO����F�еIJ��������� ��
(4) װ��ͼ�У�����F�ķ�ʽ�� ����ѡ���¶ȼ�G�����̱�ʾ��ȷ����__________������ţ���
| A��0�桫50�� | B��0�桫100�� | C��0�桫200�� | D��0�桫360�� |
(1) NaOH��Һ����CuSO4��Һ����1�֣�
��ȥH2S��AsH3��PH3��Щ�������壬��ֹ�����ж�����2�֣�
(2) ��ȴ���������ɵ���ȩ��������2�֣�
(3) ��������ˮ��Ũ���� ��1�֣�
ȡһֽ�ۣ���������HgOҩƷ��Ȼ��������ƿƽ�ź�ֽ������������ƿ�ڣ���������ֽ����ҩƷ���뼴�ɣ���2�֣�
(4) ˮԡ���ȣ���1�֣� B ��1�֣�
(5) ��ȡ����������Һ��ȡ������һ�ྻ���Թ��У�������μ��Թ�D�е�Һ��������ˮԡ���ȣ����Թ��ڱ��й����������ɣ���֤����ȩ���Ƴ�����3�֣�
��ȥH2S��AsH3��PH3��Щ�������壬��ֹ�����ж�����2�֣�
(2) ��ȴ���������ɵ���ȩ��������2�֣�
(3) ��������ˮ��Ũ���� ��1�֣�
ȡһֽ�ۣ���������HgOҩƷ��Ȼ��������ƿƽ�ź�ֽ������������ƿ�ڣ���������ֽ����ҩƷ���뼴�ɣ���2�֣�
(4) ˮԡ���ȣ���1�֣� B ��1�֣�
(5) ��ȡ����������Һ��ȡ������һ�ྻ���Թ��У�������μ��Թ�D�е�Һ��������ˮԡ���ȣ����Թ��ڱ��й����������ɣ���֤����ȩ���Ƴ�����3�֣�
��

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
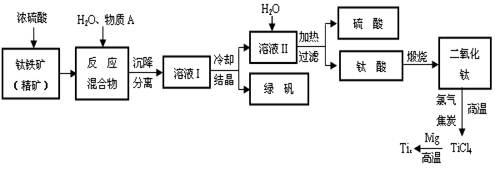
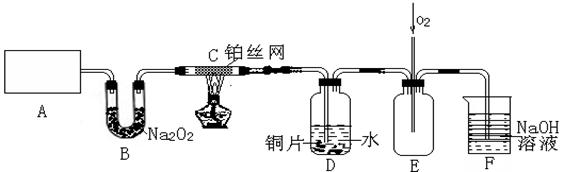 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�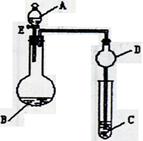
 _____________��
_____________��
 (OH)2(����) 10.8g(NH4)2SO4
(OH)2(����) 10.8g(NH4)2SO4
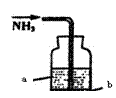
 ____________��
____________��