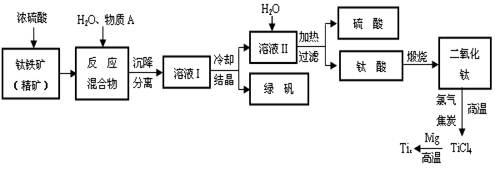
��Ŀ����
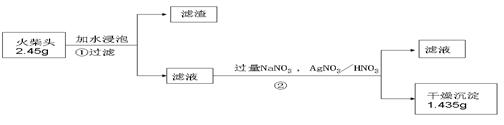
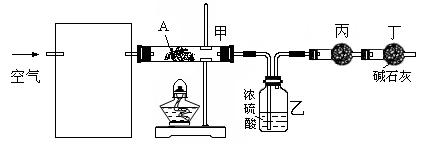
��14�֣���һ��ѧ��ƷNa2SO3�����ܺ���NaCl��Na2SO4��KNO3��K2CO2��K2SO4�е�һ�ֻ������ʣ�ijʵ��С������ͼ12�ṩ��װ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30g������6.0mol��L-1��������������������ɫ����560mL����״���������ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ����ش��������⣺
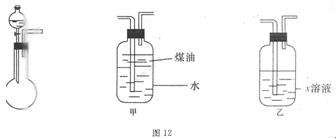
��1����ҺX�� ��ú�͵������� ��
��2����ʵ���м���������Һ�����Ϊ5.00mL������Ʒ��Na2SO3������������ ��
д���й����ӷ���ʽ ��
��3��������ṩ��ʵ�����������ʵ�飨ʵ��������ѡ�������ȷ������������������������裺
��
��4��˵��һ�����������Ƶ����ݣ�
 ��
��

��1����ҺX�� ��ú�͵������� ��
��2����ʵ���м���������Һ�����Ϊ5.00mL������Ʒ��Na2SO3������������ ��
д���й����ӷ���ʽ ��
��3��������ṩ��ʵ�����������ʵ�飨ʵ��������ѡ�������ȷ������������������������裺
��
��4��˵��һ�����������Ƶ����ݣ�
 ��
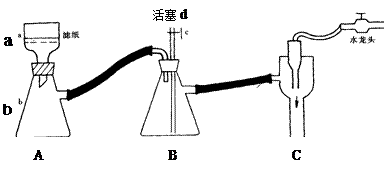
����1��X��Һ��ŨH2SO4��1�֣��������Ƿ�ֹSO2��ˮ�Ӵ���1�֣� ���������𰸲��ո��֣�
���������𰸲��ո��֣�
��2��50%��2�֣�SO32-+2H+=SO2��+H2O��3�֣� Ba2++SO42-=BaSO4�� ��2�֣�
��3����Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ����������������𰸲��ո��֣�3�֣�
��4���������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol �����Ա��������ƣ����������𰸲��ո��֣�2�֣�
 ���������𰸲��ո��֣�
���������𰸲��ո��֣���2��50%��2�֣�SO32-+2H+=SO2��+H2O��3�֣� Ba2++SO42-=BaSO4�� ��2�֣�
��3����Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ����������������𰸲��ո��֣�3�֣�
��4���������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol �����Ա��������ƣ����������𰸲��ո��֣�2�֣�
��

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ

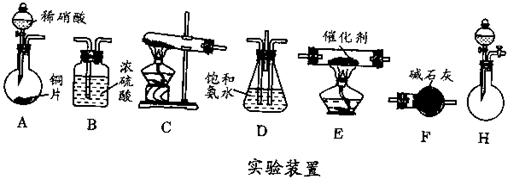
 �ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
�ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�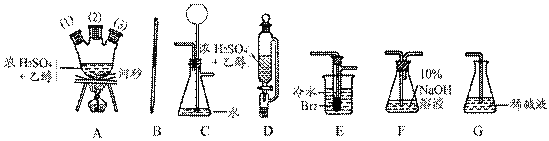
 ____________________��
____________________�� �飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������
�飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������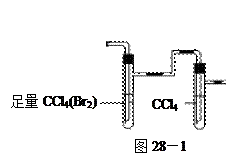 ����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
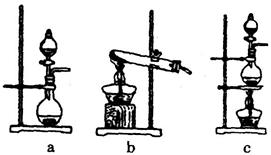
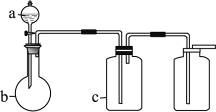
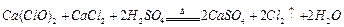 ��ijѧϰС�����ô�ԭ����
��ijѧϰС�����ô�ԭ���� ����ͼ��ʾװ����ȡ������̽�������ʡ�
����ͼ��ʾװ����ȡ������̽�������ʡ�

 ����װ����FeCl2��ҺCl2��Ӧ�����ӷ���ʽ����������������֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���ǣ���
����װ����FeCl2��ҺCl2��Ӧ�����ӷ���ʽ����������������֤��FeCl2��Cl2������������ԭ��Ӧ��ʵ�鷽���ǣ��� ���Լ������ơ���������֣�����������������������
���Լ������ơ���������֣�����������������������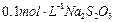 ��Һ��
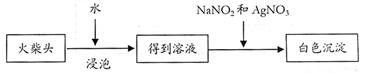
��Һ�� ��������20mLNa2S2O3��Һ����Ư����Ca(ClO)2����������Ϊ��������������������
��������20mLNa2S2O3��Һ����Ư����Ca(ClO)2����������Ϊ�������������������� n��ֵ�����������̽���ʵ�飺
n��ֵ�����������̽���ʵ�飺
 _____________��
_____________��
 (OH)2(����) 10.8g(NH4)2SO4
(OH)2(����) 10.8g(NH4)2SO4
 ____________��
____________��