��Ŀ����
2�������й��������жϻ��ʾ������ȷ���ǣ�������| A�� | ��C��s��ʯī���TC��s�����ʯ����H=+1.9 kJ•mol-1����֪ʯī�Ƚ��ʯ���ȶ� | |
| B�� | ���������������������ֱ���ȫȼ�գ����߷ų����������� | |
| C�� | ��H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ•mol-1����֪��1 mol CH3COOH����Һ�뺬1 mol NaOH����Һ��ֻ�Ϸ�Ӧ���ų�����������57.3 kJ | |
| D�� | 2g H2��ȫȼ������Һ̬ˮ�ų�285.8 kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ2H2��g��+O2��g���T2H2O��l����H=+571.6 kJ•mol-1 |
���� A�����ʾ��е�����Խ��Խ�ȶ���
B�������ת��Ϊ�������Ĺ��������ȸù��̣����ȼ�չ����Ƿ��ȹ��̣�
C�����������ᣬ������������ȹ��̣�
D�������Ȼ�ѧ����ʽ���������д�������жϣ�
��� �⣺A����C��ʯī���TC�����ʯ����H=+1.9kJ/mol����֪ʯī���е������ϵͣ����ʾ��е�����Խ��Խ�ȶ�������ʯī�Ƚ��ʯ���ȶ�����A��ȷ��
B�������ת��Ϊ�������Ĺ��������ȸù��̣��������������ֱ���ȫȼ�գ��������ų��������࣬��B����
C�����������ᣬ������������ȹ��̣���1mol CH3COOH����Һ�뺬1mol NaOH��ϡ��Һ��ϣ��ų�����С��57.3 kJ����C����
D��2gH2��1molH2��ȫȼ������Һ̬ˮ�ų�285.8kJ��������2molH2ȼ�շų�������285.8kJ��2=571.6KJ��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2 H2O��l����H=-571.6kJ/mol����D����
��ѡA��
���� ���⿼�黯ѧ��Ӧ�ķ�Ӧ�Ⱥ��ʱ��Ĺ�ϵ֪ʶ��ע���к��ȵIJⶨʵ����ʹ�õ���ǿ���ǿ���ϡ��Һ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
17��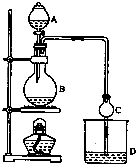 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
��ش�
��1��Ũ��������������ԡ���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��CH3CO18OH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H218O��
��2�����θ����C�������Ƿ�ֹ����������������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ����Ӧ������D�е���������Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz��
��3�����÷�Һ���������ƣ����� D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ȼ�������ˮ�Ȼ��ƣ�������Ҵ����ټ��루������ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եýϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
 ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪��
ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ��A�з���Ũ���ᣬB�з����Ҵ�����ˮ�����ƣ�D�з��б���̼������Һ����֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2•6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��1��Ũ��������������ԡ���������ˮ��������ͬλ��18Oʾ�ٷ�ȷ����Ӧ����ˮ��������ԭ�ӵ��ṩ�ߣ�д���ܱ�ʾ18Oλ�õĻ�ѧ����ʽ��CH3CO18OH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H218O��
��2�����θ����C�������Ƿ�ֹ����������������Ӧǰ��D�м��뼸�η�̪����Һ�ʺ�ɫ����Ӧ������D�е���������Һ�ֲ㣬�ϲ���ɫ����Һ�壬�²���Һ��ɫ��dz��
��3�����÷�Һ���������ƣ����� D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ȼ�������ˮ�Ȼ��ƣ�������Ҵ����ټ��루������ѡ����ѡ��C��Ȼ����������ռ�77�����ҵ���֣��Եýϴ���������������
A������������ B����ʯ�� C����ˮ������ D����ʯ�ң�
7��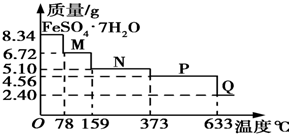 8.34g FeSO4•7H2O��Ʒ�ڸ�������������������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ������˵������ȷ���ǣ�������
8.34g FeSO4•7H2O��Ʒ�ڸ�������������������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ������˵������ȷ���ǣ�������
 8.34g FeSO4•7H2O��Ʒ�ڸ�������������������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ������˵������ȷ���ǣ�������
8.34g FeSO4•7H2O��Ʒ�ڸ�������������������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �¶�Ϊ78��ʱ��������M�Ļ�ѧʽΪFeSO4•5H2O | |
| B�� | �¶�Ϊ159��ʱ��������N�Ļ�ѧʽΪFeSO4•3H2O | |
| C�� | �ڸ���������������N�õ�P�Ļ�ѧ����ʽΪFeSO4�TFeO+SO3�� | |
| D�� | ȡ����380��ʱ���õ���ƷP����������������650�棬�õ�һ�ֹ�������Q��ͬʱ��������ɫ�������ɣ�Q�Ļ�ѧʽΪFe2O3 |
14���ֱ���Ԫ�����ڱ���2��3���ڵ�����Ԫ��A��B���������ӵĺ�����Ӳ�������㣮��֪A���ڵ�m�壬B���ڵ�n�壬��Aֻ�������ϼۣ���A��B��Ԫ�ص�ԭ�������ֱ�Ϊ��������
| A�� | m��n | B�� | m-2��10-n | C�� | m+2��n+10 | D�� | m+8��n+10 |
 ��
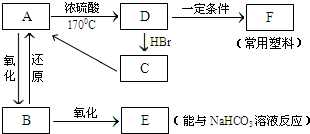
��
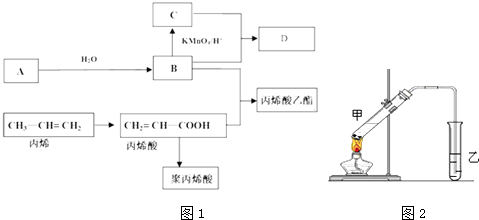
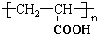 ��
��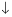 +2NO
+2NO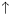 +4H+
+4H+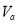 ��
��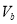 ����
����