��Ŀ����
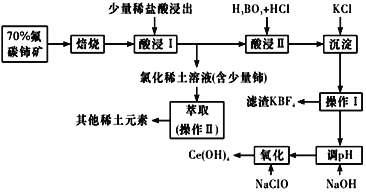
����Ŀ��ʪ�����ֳ�Ϊ����������������������Һ��ͨ����������������ͬʱ�õ������ԭ����ͼ��ʾ��ͼ��HA��ʾ������ӣ�A����ʾ��������ӣ��� ����˵����ȷ���ǣ� ��.
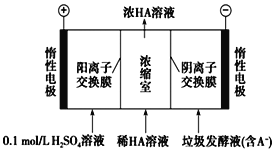
A.����11.2LO2����ʱ����·��ͨ��2 mol e���ĵ���
B.���ӴӸ������������Һ�ص�����
C.ͨ���A��ͨ�������ӽ���Ĥ������������Ũ����
D.ͨ�����������pH����
���𰸡�C
��������
A. ��֪���Ƿ��ڱ�״���£������㣬��A����
B. ���ӴӸ��������ص����������ܾ����������Һ����B����
C. ͨ���A��ͨ�������ӽ���Ĥ������������Ũ���ң���C��ȷ��
D. ͨ��������������ŵ��Ϊ������ʣ�������ӣ������������pH��С����D����
������������ΪC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ