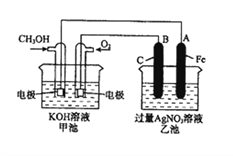
��Ŀ����
����Ŀ����֪�л���A��B��C��D��E��F������ת����ϵ��A�IJ������Ժ���һ�����ҵ�ʯ�ͻ�����չˮƽ��E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����FΪ�߷��ӻ���������ͼ��ϵ�ش����⣺
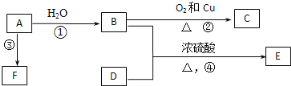
��1��д��C�Ľṹ��ʽ��___________��
��2��д��B��D�й����ŵ����ƣ�B____________��D_____________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��_________________________��
��________________________��
��4������B����ֱ�ӱ�����ΪD���������Լ���____________________________��
���𰸡�CH3CHO�ǻ��Ȼ�2CH3CH2OH+O2![]() 2CH3CHO+2H2OCH3COOH+CH3CH2OH
2CH3CHO+2H2OCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���Ը��������Һ���������ظ������Һ��
CH3COOCH2CH3+H2O���Ը��������Һ���������ظ������Һ��
��������
A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ�ı�־����AΪCH2=CH2��CH2=CH2��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH��CH3CH2OH��Cu�����������·�������������CΪCH3CHO��E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2������EΪCH3COOC2H5��DΪCH3COOH����ϩ�����Ӿ۷�Ӧ����FΪ![]() ���ݴ˽��
���ݴ˽��
�������Ϸ�����֪AΪCH2=CH2��BΪCH3CH2OH��CΪCH3CHO��DΪCH3COOH��EΪCH3COOC2H5��FΪ![]() ����
����
��1��C����ȩ���ṹ��ʽΪCH3CHO��
��2��BΪCH3CH2OH�����еĹ�����Ϊ�ǻ���DΪCH3COOH�����еĹ�����Ϊ�Ȼ���
��3����Ӧ�ڵĻ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ�ܵĻ�ѧ����ʽΪCH3COOH+CH3CH2OH
2CH3CHO+2H2O����Ӧ�ܵĻ�ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4���Ҵ�����ֱ������Ϊ���ᣬ��Ҫ����ǿ����������˼�����Լ�����������KMnO4��Һ��������K2Cr2O4��Һ����

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�����Ŀ��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��1��һ���¶������ں����ܱ�������N2O5�ɷ������з�Ӧ��N2O5(g)![]() 4NO2(g)+O2(g)����H>0
4NO2(g)+O2(g)����H>0
�����Ϊ��Ӧ��T1 �¶��µIJ���ʵ��������
t/s | 0 | 500 | 1000 |
c(N2O5)/mol��L-1 | 5.00 | 3.52 | 2.48 |
��500s��N2O5�ķֽ�����Ϊ____________________��
����T 2�¶��£���Ӧ1000sʱ���NO2��Ũ��Ϊ4.98 mol��L-1����T2________T1( ��>��<��=)��
��2������H2��O2������Na2CO3��ɵ�ȼ�ϵ�ز��õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��
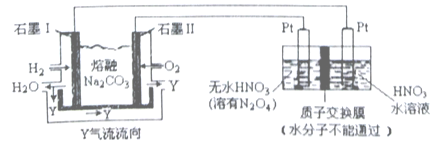
д��ʯī���缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ_____________________��N2O5 �ڵ��ص�_____________������(��������������������)��