��Ŀ����
�������ƣ�NaClO2����һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǡ���֬��Ư����ɱ���������ǹ������ⷨ�����������ƵĹ�������ͼ��
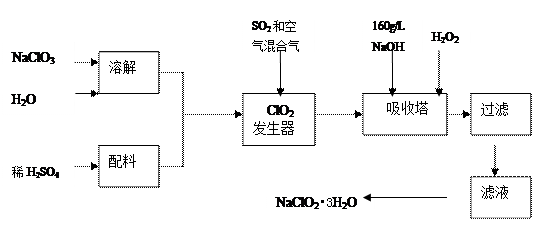

��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2?3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10�����°�ȫ��
��160 g/L NaOH��Һ��ָ160 gNaOH��������ˮ������Һ�����Ϊ1L��
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ ��������Ҫ�������Һ������������
����Ҫ��һ�������������������� ��������������������˵������
��2���������й�����������ÿ�������������ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը��c����NaClO3������ClO2
��3���������ڵķ�Ӧ�Ļ�ѧ����ʽΪ��������������������������������������
���������¶Ȳ��ܳ���20�棬��Ŀ�������� ��
��4���ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH��
������ļ�ʵ�鷽���� ��
��5����������Ϊ��ֹNaClO2����ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2�⣬������ѡ��Ļ�ԭ������ ������ѡ����ţ���
a��Na2O2 b��Na2S c��FeCl2
��6������Һ�еõ�NaClO2?3H2O�־����ʵ�����������������������ѡ����ţ���
a������ b������ c������ d������ e����ȴ�ᾧ
Ҫ�õ�������NaClO2?3H2O���������еIJ��������������� ��������������ƣ���


��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2?3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10�����°�ȫ��
��160 g/L NaOH��Һ��ָ160 gNaOH��������ˮ������Һ�����Ϊ1L��
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ ��������Ҫ�������Һ������������
����Ҫ��һ�������������������� ��������������������˵������
��2���������й�����������ÿ�������������ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը��c����NaClO3������ClO2
��3���������ڵķ�Ӧ�Ļ�ѧ����ʽΪ��������������������������������������
���������¶Ȳ��ܳ���20�棬��Ŀ�������� ��
��4���ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH��
������ļ�ʵ�鷽���� ��
��5����������Ϊ��ֹNaClO2����ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2�⣬������ѡ��Ļ�ԭ������ ������ѡ����ţ���
a��Na2O2 b��Na2S c��FeCl2
��6������Һ�еõ�NaClO2?3H2O�־����ʵ�����������������������ѡ����ţ���
a������ b������ c������ d������ e����ȴ�ᾧ
Ҫ�õ�������NaClO2?3H2O���������еIJ��������������� ��������������ƣ���
��1����4mol/L��1�֣�δд��λ�����֣�������Һ���ܶȣ�1�֣���
��2��b��2�֣���
��3��2NaOH+2ClO2+H2O2��2NaClO2+2H2O2+O2��2�֣�����ֹH2O2�ֽ⣨1�֣���
��4�������ⶨ����������Һ��pHֵ��2�֣�����5��a ��1�֣���
��6��b��e��d��2�֣����ؽᾧ��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 O
O  Na2CO3
Na2CO3 ��CO2����H2O
��CO2����H2O  ��������Һ�����Ƚᾧ����
��������Һ�����Ƚᾧ���� ��ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ƶķ���ʵ�顣
��ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ƶķ���ʵ�顣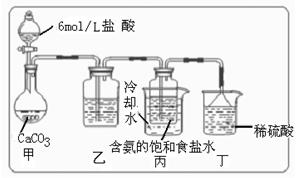
 ������װ���е��Լ���
������װ���е��Լ��� 
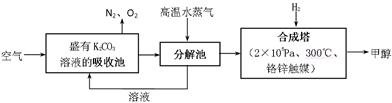
 8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ ��
8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ �� 2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ�����
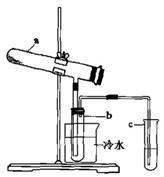
2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ����� Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ�
Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ� ��
��
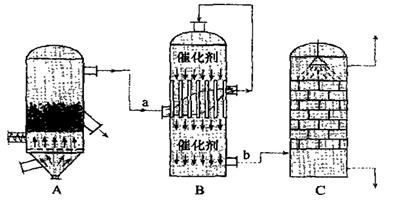
 Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת����
Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� ���� ���� ���ɲ���������
���� ���� ���ɲ���������