题目内容
【题目】滴定实验是化学学科中重要的定量实验。请回答下列问题:
I.酸碱中和滴定——用标准盐酸滴定未知浓度的NaOH溶液。
(1)该学生的实验操作如下:
a.用碱式滴定管取稀NaOH 25.00 mL,注入锥形瓶中,加入甲基橙作指示剂。
b.用待测定的溶液润洗碱式滴定管。
c.用蒸馏水洗干净滴定管。
d.取下酸式滴定管用标准的HCl溶液润洗后,将标准液注入滴定管刻度“0”以上2~3 cm处,再把滴定管固定好,调节液面至刻度“0”或“0”刻度以下。
e.检查滴定管是否漏水。
f.另取锥形瓶,再重复操作一次。
g.把锥形瓶放在滴定管下面,瓶下垫一张白纸,边滴边摇动锥形瓶直至滴定终点,记下滴定管液面所在刻度。
①滴定操作的正确顺序是(用序号填写)___________________。
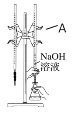
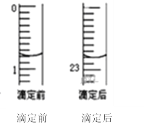
②某次滴定前、后的盛放盐酸滴定管中液面的位置。请回答:
仪器A的名称是_____________;盐酸的体积读数:滴定前读数为____mL,滴定后读数为____mL;
③在g操作中如何确定终点________________。
(2)下列操作造成测定结果偏高的是______(填选项字母)。
A.滴定终点时,俯视滴定管溶液液面
B.盛装未知液的锥形瓶用蒸馏水洗过,未用未知液润洗
C.酸式滴定管用蒸馏水洗净后,未用标准盐酸润洗
D.滴定前,滴定管尖嘴有气泡,滴定后气泡消失
II.氧化还原滴定——取草酸溶液置于锥形瓶中,加入适量稀硫酸,用浓度为0.1 mol/L的高锰酸钾溶液滴定,发生的反应为:2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2↑+2MnSO4+8H2O。表格中记录了实验数据:
滴定次数 | 待测液体积(mL) | 标准KMnO4溶液体积(mL) | |
滴定前读数 | 滴定后读数 | ||
第一次 | 25.00 | 0.50 | 20.40 |
第二次 | 25.00 | 3.00 | 23.00 |
第三次 | 25.00 | 4.00 | 24.10 |
(1)滴定时,KMnO4溶液应装在___(“酸”或“碱”)式滴定管中,滴定终点时锥形瓶内的颜色变化是___。
(2)该草酸溶液的物质的量浓度为__________。
【答案】e、c、b、a、d、g、f 酸式滴定管 0.80 22.80 当滴入最后一滴溶液,锥形瓶内由黄色变为橙色且半分钟内不褪色 CD 酸 无色变为浅紫色,且半分钟内不褪色 0.2 mol/L
【解析】
I.(1)①滴定实验步骤有检漏、洗涤、润洗、装液、取待测液加指示剂、滴定等操作;
②根据仪器结构判断仪器名称,根据滴定管小刻度在上,大刻度在下,结合滴定前后液体凹液面位置读数;
③根据用甲基橙作指示剂时,溶液由黄色变橙色,且半分钟内不变色,则到达滴定终点;
(2)根据操作对消耗标准溶液体积大小,结合溶液的物质的量浓度定义式分析实验误差;
II.(1)根据高锰酸钾溶液具有强氧化性选择滴定管类型;根据滴定结束前溶液为无色,滴定结束时溶液变成紫红色为滴定终点;
(2)先判断滴定数据的有效性,然后计算出消耗标准液的平均体积,再根据c(待测)=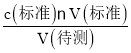 分析计算出待测液的浓度。
分析计算出待测液的浓度。
I.(1)①中和滴定操作步骤有检漏、洗涤、润洗、装液、取待测液并加指示剂、滴定等操作,所以滴定操作的正确顺序是e、c、b、a、d、g、f;
②仪器A为下部有玻璃活塞,因此为酸式滴定管,结合滴定管结构可知:在滴定前读数为0.80 mL,滴定后读数为22.80 mL;
③用甲基橙作指示剂时,溶液由黄色变橙色,且半分钟内不变色,则到达滴定终点;
(2) A.滴定终点读数时,俯视滴定管的刻度,造成V(标准)偏小,根据c(待测)=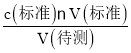 ,可以知道c(待测)偏小,A不符合题意;
,可以知道c(待测)偏小,A不符合题意;
B.盛装未知液的锥形瓶用蒸馏水洗过,未用未知液润洗,对V(标准)无影响,根据c(待测)=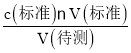 ,可以知道c(待测)不变,B不符合题意;
,可以知道c(待测)不变,B不符合题意;
C.酸式滴定管用蒸馏水洗净后,未用标准盐酸润洗,标准盐酸浓度偏小,造成V(标准)偏大,导致c(待测)偏大,C符合题意;
D.滴定前,滴定管尖嘴有气泡,滴定后气泡消失,造成V(标准)偏大,最终导c(待测)偏大,D符合题意;
故合理选项是CD;
II.(1)酸性高锰酸钾溶液具有强氧化性,能够氧化碱式滴定管的橡胶管,所以应该使用酸式滴定管盛装酸性高锰酸钾溶液;滴定结束前混合液为无色,滴定结束时混合液变成了浅紫色,所以滴定终点现象为:锥形瓶中溶液由无色变为浅紫色,且半分钟内不褪色;
(2)三次滴定消耗标准液盐酸体积分别为:(20.400.50)mL=19.90 mL、(23.003.00)mL=20.00m L、(24.104.00)mL=20.10 mL,所以三次滴定的数据都有效,则消耗标准液的平均体积为V=![]() =20.00 mL,高锰酸钾的物质的量为n(KMnO4)=cV=0.10 mol/L×0.020 L=0.0020 mol,根据反应2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2↑+2MnSO4+8H2O可知:n(H2C2O4)=
=20.00 mL,高锰酸钾的物质的量为n(KMnO4)=cV=0.10 mol/L×0.020 L=0.0020 mol,根据反应2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2↑+2MnSO4+8H2O可知:n(H2C2O4)=![]() n(KMnO4)= 0.005 mol,故待测液草酸的物质的量浓度c(H2C2O4)=
n(KMnO4)= 0.005 mol,故待测液草酸的物质的量浓度c(H2C2O4)=![]() =0.2 mol/L。
=0.2 mol/L。