��Ŀ����
2��2008��5��12��14ʱ28�֣��ҹ��Ĵ�ʡ�봨�����ش���𣬸������������������˾����ѣ��������ᣨCH3COOOH������������������Ϊʹ�õ���������������H2O2�ͱ����ᷴӦ��ȡ�������ڹ��������г����в�����H2O2���ⶨ��Ʒ�й�������Ũ��c1���漰���з�Ӧ����2MnO4-+5H2O2+6H+�T2Mn2++5O2ʮ8H2O
��H2O2+2I-+2H+�TI2+2H2O
��CH3COOOH+2I-+2H+�TCH3COOH+I2+H2O
��I2+2S2O32-�T2I-+S4O62-
��ش��������⣺
��l����ƽ��Ӧ�ٵ����ӷ���ʽ���������ת�������
2MnO4-+5H2O2+6H+�T2Mn2++5O2+8H2O
��2����Na2S2O3����Һ�ζ�I2ʱ����Ӧ�ܣ�ѡ�õ�ָʾ���ǵ�����Һ����ζ��յ�ʱ��Һ����ɫ����ɫ��
��3��ȡb0mL����Һ��������ʹ��Һ�ữ������Ũ��Ϊa1mol•L-1��KMnO4����Һ�ζ����е�H2O2�����õ�KMnO4���Ϊb1mL����Ӧ�٣��ζ�������KMnO4����������ᷴӦ����
��ȡb0mL����Һ�����������KI����������ʹ��Һ�ữ����ʱ��������Ͳ�����H2O2���ܸ� KI��Ӧ����I2����Ӧ�ںۣ͢�������Ũ��Ϊa2mol•L-1��Na2S2O3����Һ�ζ����ɵ�I2������Na2S2O3��Һ���Ϊb2mL��
���������ʵ�����ݼ�����������Ũ�ȣ��ú�a1��a2��b0��b1��b2�Ĵ���ʽ��ʾ����c0=$\frac{{{a_2}{b_2}-5{a_1}{b_1}}}{{2{b_0}}}$��
��4��Ϊ�������Һ�й��������Ũ��c0�������KI�������ѹ�����û��ȷ�������Ƿ�Ӱ��ⶨ��������ǻ��
���� ��1��������ӦԪ�ػ��ϼ۱仯������������ԭ��Ӧ��ʧ�����غ���ԭ�Ӹ����غ���ƽ����ʽ��
��2�����������۱���ɫ��Na2S2O3��ⷢ��������ԭ��Ӧʹ��Һ��ɫ��
��3�����ݸ�����غ�˫��ˮ֮��Ĺ�ϵ����˫��ˮ�����ʵ���Ũ�ȣ�������������Ƽ��������ʵ���������˫��ˮ�͵�֮��Ĺ�ϵʽ����˫��ˮ�������ɵĵ�����ʵ�������ʣ�����ǹ��������������������ɵĵ⣬���ݵ��������֮��Ĺ�ϵʽ�����������Ũ�ȣ�
��4��������������͵ⵥ�ʵ����йأ�Ϊ��֤���еĹ�������ȫ��Ӧ�������õ⻯�ع��������ݵõ����ʵ������ȷ���������������
��� �⣺��1����Ӧ�У���Ԫ�ش�+7�۽�Ϊ+2�ۣ�˫��ˮ�е���Ԫ�ش�-1������Ϊ�����е�0�ۣ�Ҫʹ��ʧ�����غ㣬�����������ϵ��Ϊ2��˫��ˮϵ��Ϊ5�����ԭ�Ӹ����غ㣬��Ӧ����ʽ��2 MnO4-+5H2O2+6H+�T2 Mn2++5O2ʮ8H2O��
�ʴ�Ϊ��2��5��6H+��2��5��8��
��2���ⵥ����������Һ��������Na2S2O3����Һ�ζ�I2ʱѡ�õ�ָʾ��Ϊ���ۣ�����Na2S2O3����Һ�ĵ��룬��ɫ��ȥ��
�ʴ�Ϊ��������Һ�������ޣ�
��3�����ݷ�ӦI2+2S2O32-�T2I-+S4O62-��֪����������Ͳ�����H2O2��KI��Ӧ����I2��������$\frac{{a}_{2}{b}_{2}��1{0}^{-3}}{2}$
mol��
���ݷ�Ӧ2MnO4-+5H2O2+6H+�T2Mn2++5O2+8H2O����ϸ�����ص����ʵ������Լ���˫��ˮ�����ʵ���
$\frac{5{a}_{1}{b}_{1}��1{0}^{-3}}{2}$mol��
���ݷ�ӦH2O2+2I-+2H+�TI2+2H2O����֪��˫��ˮ�������������ɵⵥ�ʵ����ʵ���Ϊ$\frac{5{a}_{1}{b}_{1}��1{0}^{-3}}{2}$mol��
���Թ��������������������ɵⵥ�ʵ����ʵ���Ϊ����$\frac{{a}_{2}{b}_{2}��1{0}^{-3}}{2}$-$\frac{5{a}_{1}{b}_{1}��1{0}^{-3}}{2}$��mol��
���ݷ�ӦCH3COOOH+2I-+2H+�TCH3COOH+I2+H2O
1 1
��֪������������ʵ���Ϊ��$\frac{{a}_{2}{b}_{2}��1{0}^{-3}}{2}$-$\frac{5{a}_{1}{b}_{1}��1{0}^{-3}}{2}$��mol����Һ�����Ϊb0mL����b0��10-3L��
������Ũ��Ϊ��c=$\frac{n}{V}$=$\frac{{a}_{2}{b}_{2}-5{a}_{1}{b}_{1}}{2{b}_{0}}$��
�ʴ�Ϊ��$\frac{{{a_2}{b_2}-5{a_1}{b_1}}}{{2{b_0}}}$��
��4�����ݣ�3���ļ�����Ա����������Һ�й��������Ũ��c0������KI�����������أ�
�ʴ�Ϊ����
���� ���⿼����������ԭ��Ӧ�����������ԭ����ʽ��ƽ���йؼ��㣬��ȷ����Ӧ��֮�����Ĺ�ϵ�ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ���ۺ���ά�� | B�� | ����ͱ�ϩ�� | ||
| C�� | �������������Ӳ֬������� | D�� | �������������� |
| A�� | �� | B�� | �٢� | C�� | �٢ܢ� | D�� | �٢ۢܢ� |
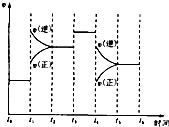 ��ͼ��ʾ��ӦN2��g��+3H2��g��2NH2��g����H��0����ij��ʱ��t0��t6�з�Ӧ�����뷴Ӧ���̵�����ͼ���İٷֺ�����ߵ�һ��ʱ���ǣ�������
��ͼ��ʾ��ӦN2��g��+3H2��g��2NH2��g����H��0����ij��ʱ��t0��t6�з�Ӧ�����뷴Ӧ���̵�����ͼ���İٷֺ�����ߵ�һ��ʱ���ǣ�������| A�� | t5��t6 | B�� | t2��t3 | C�� | t3��t4 | D�� | t0��t1 |
| A�� | CO${\;}_{3}^{2-}$ | B�� | HCO${\;}_{3}^{-}$ | C�� | CH3COO- | D�� | SO${\;}_{3}^{2-}$ |

| A�� | ����ͼ�ٿ��жϿ��淴Ӧ��A2��g��+3B2��g��?2AB3��g�����ġ�H��0 | |
| B�� | ͼ�ڿ��Ա�ʾѹǿ�Կ��淴Ӧ��2A��g��+2B��g��?3C��g��+D��s������Ӱ�죬�ҵ�ѹǿ�� | |
| C�� | ͼ�ۿɱ�ʾ������Һ�еμ�����������Һ��������������Һ�����Եı仯 | |
| D�� | ͼ����N2��H2�ϳɰ��������仯���ߣ���ȷ���÷�Ӧ��1molN2��3molH2��ַ�Ӧʱ����һ������92kJ |
| A�� | �谱����C��H��O��N����Ԫ����� | |
| B�� | ÿ���谱����Ӻ���26��ԭ�� | |
| C�� | �谱����̼Ԫ������Ԫ�ص�������Ϊ6��1 | |
| D�� | ÿ���谱������к���1��N2���� |
 ʵ����ͨ�������Ȼ�狀���ʯ������ȡ������
ʵ����ͨ�������Ȼ�狀���ʯ������ȡ������