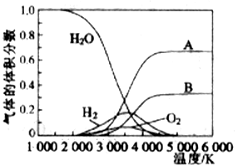
��Ŀ����
14��������Ԫ��A��B��C��D��Eԭ������������������A��B���γ�A2B2��A2B���ֻ����C��Aλ��ͬһ���壻D��B�γɵĻ������Ǵ�����Ⱦ������γ����꣮��ش��������⣺
��1����A��B��E����Ԫ�ذ�ԭ�Ӹ�����1��1��1��ɵĻ�����ĽṹʽΪH-O-Cl��
��2��д��DB2ʹ����KMn04��Һ��ɫ�����ӷ���ʽ5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+
��3�����100mL 1mol��L-l�Ļ�����CE����Һʱ����������������ʯī���缫�����һ��ʱ�䣬����������1 1.2mL���壨��״��������ʱ��Һ��p H=12��������Һ������䣩������������Ԫ���е�����Ԫ����ɣ���ԭ�Ӹ�����Ϊ1��1��1��ǿ�����x����ˮ������ˮ�ĵ��룬��x�Ļ�ѧʽΪNaOH��
��4����������DB2ͨ�뻯����C2D����Һ�У���ַ�Ӧ����Һ���ֻ��ǣ���1.2n moi������C2D����Һ��������ջ�����DB2�����ʵ���Ϊ3n mol��������ˮ���ܽ�Ļ�����DB2����
���� ������Ԫ��A��B��C��D��Eԭ��������������D��B�γɵĻ������Ǵ�����Ⱦ������γ����꣬��DΪ��Ԫ�ء�BΪ��Ԫ�أ�E��ԭ���������EΪCl��A��B���γ�A2B2��A2B���ֻ����A���ڢ�A�壬C��Aλ��ͬһ���壬C��ԭ������������Ԫ�أ���AΪHԪ�ء�CΪNaԪ�ء�A2B2ΪH2O2��A2BΪH2O���ݴ˽��
��� �⣺������Ԫ��A��B��C��D��Eԭ��������������D��B�γɵĻ������Ǵ�����Ⱦ������γ����꣬��DΪ��Ԫ�ء�BΪ��Ԫ�أ�E��ԭ���������EΪCl��A��B���γ�A2B2��A2B���ֻ����A���ڢ�A�壬C��Aλ��ͬһ���壬C��ԭ������������Ԫ�أ���AΪHԪ�ء�CΪNaԪ�ء�A2B2ΪH2O2��A2BΪH2O��
��1����H��O��Cl����Ԫ�ذ�ԭ�Ӹ�����1��1��1��ɵĻ�����ΪHClO���ṹʽΪH-O-Cl��
�ʴ�Ϊ��H-O-Cl��
��2��SO2ʹ����KMn04��Һ��ɫ�����ӷ���ʽ��5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
�ʴ�Ϊ��5SO2+2MnO4-+2H2O=5SO42-+2Mn2++4H+��
��3�����100mL 1mol��L-�Ļ�����NaCl����Һ��NaCl���ʵ���Ϊ0.1L��1mol/L=0.1mol����������������ʯī���缫�����һ��ʱ�䣬����������11.2mL���壨��״���������������������ʵ���Ϊ$\frac{0.0112L}{22.4L/mol}$=0.0005mol����2NaCl+H2O=2NaOH+H2��+Cl2����֪����NaOHΪ0.0005mol��2=0.001mol����c��OH-��=$\frac{0.001mol}{0.1L}$=0.01mol/L����Һ��c��H+��=$\frac{1{0}^{-14}}{0.01}$mol/L=10-12mol/L����Һ��pH=-lg10-12=12��
����������Ԫ���е�����Ԫ����ɣ���ԭ�Ӹ�����Ϊ1��1��1��ǿ�����X����ˮ������ˮ�ĵ��룬��X�Ļ�ѧʽΪNaOH��
�ʴ�Ϊ��12��NaOH��
��4����������SO2ͨ�뻯����Na2S����Һ�У���ַ�Ӧ����Һ���ֻ��ǣ���Һ�����ɵ�NaOH��Ҳ���ն��������ܷ�Ӧ����ʽΪ��5SO2+2Na2S+2H2O=4NaHSO3+3S�����ʺ�1.2n mol������Na2S����Һ��������ջ�����SO2�����ʵ���Ϊ1.2n mol��$\frac{5}{2}$=3n mol��
�ʴ�Ϊ��3n mol��
���� ���⿼��Ԫ�ػ������ƶϡ����ӷ���ʽ��д����ҺpH���㡢����йؼ��㡢��ѧ����ʽ����ȣ���4���м���Ϊ�״��㡢�ѵ㣬ѧ�����Կ��Ƕ������������Ʒ�Ӧ������NaOH���Ѷ��еȣ�

| ѡ�� | ���� | ���� |
| A | H2O2��SO2����ʹ���Ը��������Һ��ɫ | ǰ�߱��ֳ���ԭ�Ժ��߱��ֳ�Ư���� |
| B | �����������ڿ����н����䰵 | ������ѧ��ʴ |
| C | SiO2��������ἰ�Ӧ | SiO2������������ |
| D | BaSO4������Һ�м��뱥��Na2CO3 ��Һ��BaCO3���� | ˵��Kvp��BaSO4����Kvp��BaSO3�� |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
| A�� | ��⾫��ͭ�����������ļ�С������������������ | |
| B�� | �ȼҵ����������Ҫ���ϼ��뺬��NaOH������ˮ | |
| C�� | �ϳɰ���ҵ��ʹ�ô���ֻ�ӿ췴Ӧ���ʲ�Ӱ�컯ѧƽ�� | |
| D�� | ���Ṥҵ��SO2��ת���ʸߴ�93.5%��98%����˽Ӵ��������β������ֱ���ŷ� |
 ��ʵ�黯ѧ��
��ʵ�黯ѧ��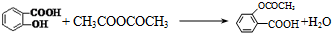
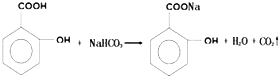 ��
��