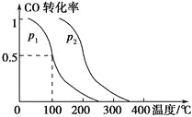
ЬтФПФкШн
ЁОЬтФПЁПвЛжжВтЖЈЫЎбљжафхРызгЕФХЈЖШЕФЪЕбщВНжшШчЯТЃК
ЂйЯђзЖаЮЦПжаМгШыДІРэКѓЕФЫЎбљ25.00mLЃЌМгШыМИЕЮNH4Fe(SO4)2ШмвКЁЃ
ЂкМгШыV1mL c1molЁЄL-1 AgNO3ШмвКЃЈЙ§СПЃЉЃЌГфЗжвЁдШЁЃ
ЂлгУc2molЁЄL-1KSCNБъзМШмвКНјааЕЮЖЈЃЌжСжеЕуЪБЯћКФБъзМШмвКV2mL ЁЃ
ЃЈвбжЊЃКKsp(AgBr)=7.7ЁС10-13,Ag++SCN-= AgSCN(АзЩЋ)Ё§ЃЌKsp(AgSCN)=1ЁС10-12ЃЉЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. ЕЮЖЈжеЕуЪБЃЌШмвКБфЮЊКьЩЋ
B. ИУЕЮЖЈЗЈашдкМюадЬѕМўЯТНјаа
C. AgBr(s)+SCNЃ![]() AgSCN(s)+Br-(aqЃЉЕФЦНКтГЃЪ§K=0.77
AgSCN(s)+Br-(aqЃЉЕФЦНКтГЃЪ§K=0.77
D. ИУЫЎбљжафхРызгХЈЖШЮЊЃКc(Br-ЃЉ=(c1V1-c2V2)/25.00mol/L
ЁОД№АИЁПB
ЁОНтЮіЁПKSCNгыЯѕЫсвјЩњГЩAgSCNГСЕэКѓЃЌдйгыFe3+ЗДгІЩњГЩFe(SCN)3ЃЌШмвКБфЮЊКьЩЋЃЌЙЪAе§ШЗЃЛдкМюадЬѕМўЯТFe3+ЩњГЩЧтбѕЛЏЬњГСЕэЃЌЙЪBДэЮѓЃЛAgBr(s)+SCNЃ![]() AgSCN(s)+Br-(aqЃЉЕФЦНКтГЃЪ§K=
AgSCN(s)+Br-(aqЃЉЕФЦНКтГЃЪ§K=![]() 0.77ЃЌЙЪCе§ШЗЃЛЫЎбљжафхРызгХЈЖШЮЊЃКc(Br-ЃЉ=(c1V1-c2V2)/25.00mol/LЃЌЙЪDе§ШЗЁЃ
0.77ЃЌЙЪCе§ШЗЃЛЫЎбљжафхРызгХЈЖШЮЊЃКc(Br-ЃЉ=(c1V1-c2V2)/25.00mol/LЃЌЙЪDе§ШЗЁЃ

 аТПЮБъНзЬндФЖСбЕСЗЯЕСаД№АИ
аТПЮБъНзЬндФЖСбЕСЗЯЕСаД№АИ ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ
ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ ЭЌВНЭиеЙдФЖСЯЕСаД№АИ
ЭЌВНЭиеЙдФЖСЯЕСаД№АИЁОЬтФПЁПвбжЊКЃЫЎЬсШЁУОЕФжївЊВНжшШчЯТЃК
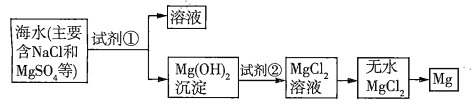
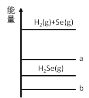
(1)ЙигкМгШыЪдМСЂйзїГСЕэМСЃЌгавдЯТЗНЗЈЃЌЧыЭъГЩЯТСаЮЪЬтЁЃ
ЗНЗЈ | ЪЧЗёКЯРэ | МђЪіРэгЩ |
ЗНЗЈЃКИпЮТМгШШеєЗЂКЃЫЎКѓЃЌдйМгШыГСЕэМС | a | b |
ФуШЯЮЊКЯРэЕФЦфЫћЗНЗЈЪЧc |
a. _____________ЃЛ
b.____________ЃЛ
c.____________ЁЃ
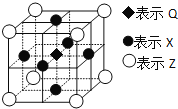
(2)ПђЭМжаМгШыЕФЪдМСЂйгІИУЪЧ_______(ЬюЮяжЪУћГЦ)ЃЌМгШыЪдМСЂкЕФШмжЪЪЧ_______(ЬюЛЏбЇЪН)ЁЃЙЄвЕЩЯгЩЮоЫЎ![]() жЦШЁУОЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ
жЦШЁУОЕФЛЏбЇЗНГЬЪНЮЊ___________ЁЃ