ЬтФПФкШн
ЁОЬтФПЁП25ЁцЃЌЯђ20.00mL 0.100 molЁЄL![]() HXжаЕЮМг0.100 molЁЄL
HXжаЕЮМг0.100 molЁЄL![]() NaOHЙ§ГЬжаЃЌpHБфЛЏШчЯТЭМЫљЪОЁЃ
NaOHЙ§ГЬжаЃЌpHБфЛЏШчЯТЭМЫљЪОЁЃ
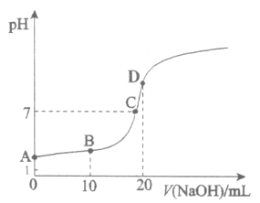
(1)аДГіHXЕФЕчРыЗНГЬЪНЃК__________ЁЃ
(2)ЯТСагаЙиBЕуШмвКЕФЫЕЗЈе§ШЗЕФЪЧ__________(ЬюзжФИађКХ)ЁЃ
a. ШмжЪЮЊЃКHXЁЂNaX
b. ЮЂСЃХЈЖШТњзуЃКc(NaЃЋ)ЃЋc(HЃЋ)ЃНc(XЃ)ЃЋc(OHЃ)
c. ЮЂСЃХЈЖШТњзуЃКc(NaЃЋ)ЃНc(HX)ЃЋc(XЃ)
d. ЮЂСЃХЈЖШТњзуЃКc(XЃ)>c(NaЃЋ)>c(HЃЋ)>c(OHЃ)
(3)AЁЂCСНЕуЫЎЕФЕчРыГЬЖШЃКA__________C(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
(4)CЕуЖдгІРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ__________ЁЃ
(5)гУРызгЗНГЬЪННтЪЭDЕуШмвКpH>7ЕФдвђЃК_______ЁЃ
ЁОД№АИЁПHXH++XЃ abd < c(NaЃЋ)ЃНc(XЃ)>c(HЃЋ)ЃНc(OHЃ) XЃ+H2OHX+OHЃ
ЁОНтЮіЁП
(1)ЙлВьЧњЯпЦ№ЕуЕФpHЃЌХаЖЯHXЪЧШѕЕчНтжЪЃЌаДГіHXЕФЕчРыЗНГЬЪНЃЛ
(2) BЕуШмвКЪЧ20.00mL 0.100 molЁЄL![]() HXжаЕЮМг0.100 molЁЄL
HXжаЕЮМг0.100 molЁЄL![]() NaOH10 mLЪБЫљЕУЃЌдђЯћКФ1ЁС10-3molHXЃЌЪЃгр1ЁС10-3molHXЃЌЩњГЩ1ЁС10-3mol NaXЃЌОнДЫХаЖЯЫЕЗЈЪЧЗёе§ШЗЃЛ
NaOH10 mLЪБЫљЕУЃЌдђЯћКФ1ЁС10-3molHXЃЌЪЃгр1ЁС10-3molHXЃЌЩњГЩ1ЁС10-3mol NaXЃЌОнДЫХаЖЯЫЕЗЈЪЧЗёе§ШЗЃЛ
(3)AЁЂCСНЕуЫЎЕФЕчРыГЬЖШПЩДгЫЎЕчРыВњЩњЕФЧтРызгЛђЧтбѕХЈЖШЕФЯрЖдДѓаЁРДБШНЯЃЛ
(4)CЕуШмвКГЪжаадЃЌНсКЯЕчКЩЪиКуПЩХаЖЯЖдгІРызгХЈЖШЕФЫГађЮЊЃЛ
(5) DЕуШмвКЮЊNaXбЮШмвКЃЌpH>7ЕФдвђЪЧвђЮЊЫЎНтЃЌОнДЫаДРызгЗНГЬЪНЁЃ
(1)ЙлВьЧњЯпЦ№ЕуЮЊ0.100 molЁЄL![]() HXШмвКЃЌpHЃО1ЃЌХаЖЯHXЪЧШѕЕчНтжЪЃЌдђHXЕФЕчРыЗНГЬЪНЮЊHXH++XЃЃЛ
HXШмвКЃЌpHЃО1ЃЌХаЖЯHXЪЧШѕЕчНтжЪЃЌдђHXЕФЕчРыЗНГЬЪНЮЊHXH++XЃЃЛ
Д№АИЮЊЃКHXH++XЃЃЛ
(2) BЕуШмвКЪЧHXжаЕЮМг10 mL 0.100 molЁЄL![]() NaOHЪБЫљЕУЃЌдђШмвКжаКЌ1ЁС10-3molHXЁЂ1ЁС10-3mol NaXЃЌЧвШмвКГЪЫсадЃЌЫЕУїЫсHXЕФЕчРыГЬЖШДѓгкбЮNaXЕФЫЎНтГЬЖШЃЌдђ
NaOHЪБЫљЕУЃЌдђШмвКжаКЌ1ЁС10-3molHXЁЂ1ЁС10-3mol NaXЃЌЧвШмвКГЪЫсадЃЌЫЕУїЫсHXЕФЕчРыГЬЖШДѓгкбЮNaXЕФЫЎНтГЬЖШЃЌдђ
a. ШмжЪЮЊЃКHXЁЂNaXЃЌЫЕЗЈе§ШЗЃЛ
b. вђЮЊШмвКжаКЌга![]() етЫФжжРызгЃЌдђЮЂСЃХЈЖШТњзуЃК
етЫФжжРызгЃЌдђЮЂСЃХЈЖШТњзуЃК![]() ЃЌШмвКжаЕчКЩЪиКуЃЌBе§ШЗЃЛ
ЃЌШмвКжаЕчКЩЪиКуЃЌBе§ШЗЃЛ
c.ШмвКжаКЌ 1ЁС10-3molHXЁЂ1ЁС10-3mol NaXЃЌАДЮяСЯЪиКужЊЃЌ2c(NaЃЋ)ЃНc(HX)ЃЋc(XЃ)ЃЌCДэЮѓЃЛ
d. ШмвКГЪЕчжаадЃЌдђ![]() ЃЌвђЮЊШмвКГЪЫсадЃЌдђc(HЃЋ)>c(OHЃ)ЃЌдђc(XЃ)>c(NaЃЋ)ЃЌЙЪЮЂСЃХЈЖШТњзуЃКc(XЃ)>c(NaЃЋ)>c(HЃЋ)>c(OHЃ)ЃЌdе§ШЗЃЛ
ЃЌвђЮЊШмвКГЪЫсадЃЌдђc(HЃЋ)>c(OHЃ)ЃЌдђc(XЃ)>c(NaЃЋ)ЃЌЙЪЮЂСЃХЈЖШТњзуЃКc(XЃ)>c(NaЃЋ)>c(HЃЋ)>c(OHЃ)ЃЌdе§ШЗЃЛ
Д№АИЮЊЃКabdЃЛ
(3)AЕуЮЊHXШмвКЃЌЫсвжжЦЫЎЕчРыЃЌCЕуШмвКГЪжаадЃЌдђЫЎЕчРыВњЩњЕФЧтРызгХЈЖШЪЧAжаЕФаЁЃЌЫЎЕФЕчРыГЬЖШЪЧAжаЕФаЁЃЛ
Д№АИЮЊЃК<ЃЛ
(4)CЕуШмвКГЪжаадЃЌдђc(HЃЋ)=c(OHЃ)ЃЌШмвКГЪЕчжаадЃЌдђ![]() ЃЌЙЪc(XЃ)=c(NaЃЋ)ЃЌдђШмвКжаЖдгІРызгХЈЖШЕФЫГађЮЊc(NaЃЋ)ЃНc(XЃ)>c(HЃЋ)ЃНc(OHЃ)ЃЛ
ЃЌЙЪc(XЃ)=c(NaЃЋ)ЃЌдђШмвКжаЖдгІРызгХЈЖШЕФЫГађЮЊc(NaЃЋ)ЃНc(XЃ)>c(HЃЋ)ЃНc(OHЃ)ЃЛ
Д№АИЮЊЃКc(NaЃЋ)ЃНc(XЃ)>c(HЃЋ)ЃНc(OHЃ)ЃЛ
(5) DЕуШмвКЮЊNaXбЮШмвКЃЌЧПМюШѕЫсбЮЫЎНтГЪМюадЃЌЙЪГЃЮТЯТpH>7ЃЌдђРызгЗНГЬЪНЮЊXЃ+H2OHX+OHЃЃЛ
Д№АИЮЊЃКXЃ+H2OHX+OHЃЁЃ

 ЬЦгЁЮФЛЏПЮЪБВтЦРЯЕСаД№АИ
ЬЦгЁЮФЛЏПЮЪБВтЦРЯЕСаД№АИ ЕМбЇгыВтЪдЯЕСаД№АИ
ЕМбЇгыВтЪдЯЕСаД№АИЁОЬтФПЁПбаОПИпаЇДпЛЏМСЪЧНтОіЦћГЕЮВЦјжаЕФ NO КЭ CO ЖдДѓЦјЮлШОЕФживЊЭООЖЁЃ
(1)вбжЊЃКC(s)+O2(g)=CO2(g) ЁїH1=-393.5 kJ/mol
C(s)+![]() O2(g)= CO(g) ЁїH2= -110.5 kJ/mol
O2(g)= CO(g) ЁїH2= -110.5 kJ/mol
N2(g)+ O2(g)=2NO(g) ЁїH3= +180.0 kJ/mol
дђЦћГЕЮВЦјЕФДпЛЏзЊЛЏЗДгІ 2NO(g)+ 2CO(g)=N2(g)+ 2CO2(g)ЕФЁїH =_______kJ/molЁЃ
(2)400ЁцЪБЃЌдкЗжБ№зАгаДпЛЏМС A КЭ B ЕФСНИіШнЛ§ЮЊ 2 L ЕФИеадУмБеШнЦїжаЃЌИїГфШыЮяжЪЕФСПОљЮЊnmolЕФNOКЭCOЗЂЩњЩЯЪіЗДгІЁЃЭЈЙ§ВтЖЈШнЦїФкзмбЙЧПЫцЪБМфБфЛЏРДЬНОПДпЛЏМСЖдЗДгІЫйТЪЕФгАЯьЃЌЪ§ОнШчЯТБэЃК
ЪБМф/min | 0 | 10 | 20 | 30 | Ёо |
AШнЦїФкбЙЧП/kPa | 75.0 | 70.0 | 65.0 | 60.0 | 60.0 |
BШнЦїФкбЙЧП/kPa | 75.0 | 71.0 | 68.0 | 66.0 | 60.0 |
ЂйгЩЩЯБэПЩвдХаЖЯДпЛЏМС __________(ЬюЁАAЁБЛђЁАBЁБ) ЕФаЇЙћИќКУЁЃ
ЂкШнЦїжаCO ЕФЦНКтзЊЛЏТЪЮЊ __________ЁЃ400ЁцЪБЃЌгУбЙЧПБэЪОЕФЦНКтГЃЪ§Kp__________(kPa)-1 (БЃСєСНЮЛаЁЪ§)ЁЃ
ЂлЦћГЕЮВЦјХХЦјЙмжаЪЙгУДпЛЏМСПЩвдЬсИпЮлШОЮязЊЛЏТЪЃЌЦфдвђЪЧ __________ЁЃ
(3)ЮЊбаОПЦјИзжаNOЕФЩњГЩЃЌ дкЬхЛ§ПЩБфЕФКубЙУмБеШнЦїжаЃЌИпЮТЯТГфШыЮяжЪЕФСПОљЮЊ 1mol ЕФЕЊЦјКЭбѕЦјЃЌЗЂЩњЗДгІ N2(g)+ O2(g)2NO(g)ЁЃ
ЂйЯТСаЫЕЗЈФмБэУїИУЗДгІвбОДяЕНЦНКтзДЬЌЕФЪЧ_________(ЬюађКХ)ЁЃ
A.2vе§(O2)= vФц(NO) B.ЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПВЛБф
C.c(N2):c(O2)=l D.ШнЦїФкЮТЖШВЛБф
ЂкЮЊМѕаЁЦНКтЛьКЯЦјжа NO ЕФЬхЛ§ЗжЪ§ЃЌ ПЩВЩШЁЕФДыЪЉЪЧ ___________ЁЃ
(4)ЖдгкЦјИзжаNЕФЩњГЩЃЌЛЏбЇМвЬсГіСЫШчЯТЗДгІРњГЬЃК
ЕквЛВН O22O Т§ЗДгІ
ЕкЖўВН O+N2NO+N НЯПьЦНКт
ЕкШ§ВН N+O2NO+O ПьЫйЦНКт
ЯТСаЫЕЗЈДэЮѓЕФЪЧ_______(ЬюБъКХ)ЁЃ
A.ЕквЛВНЗДгІВЛДгN2ЗжНтПЊЪМЃЌЪЧвђЮЊN2БШO2ЮШЖЈ
B.NЁЂO дзгОљЮЊИУЗДгІЕФДпЛЏМС
C.Ш§ВНЗДгІжаЕквЛВНЗДгІЛюЛЏФмзюДѓ
D.Ш§ВНЗДгІЕФЫйТЪЖМЫцЮТЖШЩ§ИпЖјдіДѓ