��Ŀ����
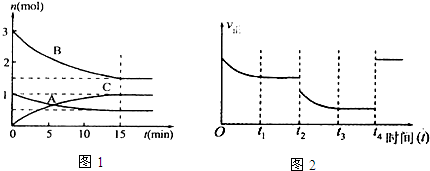
14����1����ij�ݻ�Ϊ10L���ܱ������У��п��淴Ӧ��mA��g��+nB��g��?pC��g��+qD��s����H��0����ͼ1Ϊij��Ӧ�����и��������ʵ���n��mol����ʱ��t��min���ı仯����ͼ������0��15min�ڵ�ƽ����Ӧ���ʣ�v��B��=0.01mol/��L•min����
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ$\frac{{c}^{2}��C��}{c��A��•{c}^{3}��B��}$
��2�����÷�Ӧ������Ӧ������ʱ��Ĺ�ϵ��ͼ2��ʾ���������������������£�t2ʱ�ı�����������ǽ����¶ȣ�
���� ��1����ͼ��֪��0��15min�ڡ�n��A��=0.5mol����n��B��=3mol-1.5mol=1.5mol����n��C��=1mol����m��n��p=0.5mol��1.5mol��1mol=1��3��2��
�ٸ���v=$\frac{\frac{��c}{V}}{��t}$����v��B����
�ڻ�ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��
��2����ͼ��֪���ı�������˲������Ӧ���ʽ��ͣ��������Ӧ���ʼ������ͣ�˵���ı�����ƽ��������Ӧ�����ƶ�����Ϸ�Ӧ�������з������
��� �⣺��1����ͼ��֪��0��15min�ڡ�n��A��=0.5mol����n��B��=3mol-1.5mol=1.5mol����n��C��=1mol����m��n��p=0.5mol��1.5mol��1mol=1��3��2����ӦΪA��g��+3B��g��?2C��g����
��v��B��=$\frac{\frac{1.5mol}{10L}}{15min}$=0.01mol/��L•min�����ʴ�Ϊ��0.01mol/��L•min����
��A��g��+3B��g��?2C��g����ƽ�ⳣ��k=$\frac{{c}^{2}��C��}{c��A��•{c}^{3}��B��}$���ʴ�Ϊ��$\frac{{c}^{2}��C��}{c��A��•{c}^{3}��B��}$��
��2����ͼ��֪���ı�������˲������Ӧ���ʽ��ͣ��������Ӧ���ʼ������ͣ�˵���ı�����ƽ��������Ӧ�����ƶ�����ӦA��g��+3B��g��?2C��g��������Ӧ��������ʵ�����С�ķ��ȷ�Ӧ��t2ʱ�ı������������Ϊ����ѹǿ������Ϊ�����¶ȣ��ʴ�Ϊ�������¶ȣ�
���� ���⻯ѧƽ��ͼ��ѧ��Ӧ���ʡ���ѧƽ�ⳣ����Ӱ��ƽ������صȣ���Ŀ�Ѷ��еȣ�ע����л�ѧƽ�ⳣ����д���岻��Ҫд�����Ƚ����ף�

| A�� | ��ѧ��Ӧ���ʱ仯ʱ����ѧƽ��һ�������ƶ� | |
| B�� | ��ѧƽ�ⷢ���ƶ�ʱ����ѧ��Ӧ����һ���仯 | |
| C�� | ����Ӧ���еij̶�Խ������Ӧ����һ��Խ�� | |
| D�� | ʹ��ѧ��Ӧ���ʷ����仯������ѧƽ�ⲻ�ƶ���Ӱ�����أ�һ���Ǵ��� |
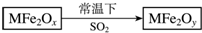 �������ײ���MFe2Ox��3��x��4����M��ʾ+2�۵Ľ���Ԫ�أ��ڷ�Ӧ�л��ϼ۲������仯�������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS��������ͼ���������ж���ȷ���ǣ�������
�������ײ���MFe2Ox��3��x��4����M��ʾ+2�۵Ľ���Ԫ�أ��ڷ�Ӧ�л��ϼ۲������仯�������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS��������ͼ���������ж���ȷ���ǣ�������| A�� | MFe2Ox�������� | B�� | SO2�Ǹ÷�Ӧ�Ĵ��� | ||
| C�� | x��y | D�� | MFe2Oy�ǻ�ԭ���� |
| A�� | 0.5s | B�� | 1s | C�� | 30s | D�� | 60s |
| A�� | ����ʹ��ú�������γ���������Ҫԭ��֮һ | |
| B�� | �ԡ��ع��͡�������Ի������ | |
| C�� | �ԷϾɵ�ؽ��л��մ�����ҪΪ�˷�ֹ�ؽ�����ȾˮԴ | |
| D�� | �뵼����ҵ����һ�仰������ɳ̲���û����������оƬ�IJ����ǹ� |
��1��2Na+2NH3=2NaNH2+H2��
��2��CaO+2NH4Cl=CaCl2+2NH3��+H2O
��3��3Mg��NH2��2$\frac{\underline{\;\;��\;\;}}{\;}$Mg3N2+4NH3��
��4��NH4Cl+NaNH2=NaCl+2NH3��
������ȷ���ǣ�������
| A�� | ֻ�У�3�� | B�� | ֻ�У�2�� | C�� | ��2���ͣ�3�� | D�� | ����ȷ |
| A�� | CO2������CaCl2��Һ��Ӧ����SO2Ҳ������CaCl2��Һ��Ӧ | |
| B�� | �������Ż�����CO2������������Ż�Ҳ������CO2���� | |
| C�� | Al2O3������ǿ���ǿ���BeOҲ������ǿ���ǿ�� | |
| D�� | Mg ��OH��2������ˮ����Ca��OH��2Ҳ������ˮ |
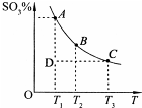 ����������������������һ�����ҵĻ���������������ҵ��������������д��ڷ�Ӧ��2SO2��g��+O2��g��?2SO3��g������Ӧ��ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
����������������������һ�����ҵĻ���������������ҵ��������������д��ڷ�Ӧ��2SO2��g��+O2��g��?2SO3��g������Ӧ��ϵ��SO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
