��Ŀ����
18��A��I�����ɶ�����Ԫ����ɵij������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪��AΪ���壬��ˮ��Һ�ʼ��ԣ�D��F����������ѪҺ�е�Ѫ�쵰��϶�ʹ���ж���E����Ϊ�뵼����ϣ�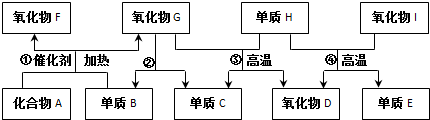
��1��������A�ĵ���ʽΪ
 ��������I�л�ѧ�����ͣ����ۼ������E���ʵ�Ԫ����Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壮ͼ����������Ԫ��ԭ�ӵİ뾶�ɴ�С�����ǣ�дԪ�ط��ţ�Si��C��N��O��H��
��������I�л�ѧ�����ͣ����ۼ������E���ʵ�Ԫ����Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壮ͼ����������Ԫ��ԭ�ӵİ뾶�ɴ�С�����ǣ�дԪ�ط��ţ�Si��C��N��O��H����2����Ӧ�ܵĻ�ѧ����ʽΪ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO����
��3����F��B�������2��1�����������M�������²ⶨM��Է�������ʱ���õ���ʵ��ֵ���DZ�����ֵƫ����ԭ���ǣ��û�ѧ����ʽ��ʾ��2NO2
 N2O4��
N2O4����4��ij�ݻ�ΪV L���Թ��г���F���壨��״���£���������������ȫ��ˮ���գ���ͨ��B�����ʵ���Ϊ��д��V�ı���ʽ��$\frac{3V}{4��22.4}$mol����ʱ������Һ����ͨ������A��ǡ����ȫ��Ӧ��������Һ�и�����Ũ���ɴ�С����Ϊ��c��NO3-����c��NH4+����c��H+����c��OH-����
���� A�����壬����ˮ��Һ�ʼ��ԣ���AӦΪNH3���뵥��B�ڴ��������·�Ӧ���������������BΪO2��������D��������F����������ѪҺ�е�Ѫ�쵰��϶�ʹ���ж�����FΪNO��DΪCO��GΪH2O�����ת����ϵ��֪CΪH2��HΪC������E�����뵼����ϣ�ӦΪSi����IΪSiO2�����ݷ�����֪��AӦΪNH3��BΪO2��CΪH2��DΪCO��EΪSi��FΪNO��GΪH2O��HΪC��IΪSiO2����϶�Ӧ���ʵ������Լ���ĿҪ��ɽ����⣮
��� �⣺A�����壬����ˮ��Һ�ʼ��ԣ���AӦΪNH3���뵥��B�ڴ��������·�Ӧ���������������BΪO2��������D��������F����������ѪҺ�е�Ѫ�쵰��϶�ʹ���ж�����FΪNO��DΪCO��GΪH2O�����ת����ϵ��֪CΪH2��HΪC������E�����뵼����ϣ�ӦΪSi����IΪSiO2�����ݷ�����֪��AӦΪNH3��BΪO2��CΪH2��DΪCO��EΪSi��FΪNO��GΪH2O��HΪC��IΪSiO2��
��1��AΪNH3��A�ĵ���ʽΪ  ��IΪSiO2��I�л�ѧ�������ǹ��ۼ���EΪSi��SiԪ����Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壬ͼ����������Ԫ��ԭ�ӵİ뾶�ɴ�С������Si��C��N��O��H��
��IΪSiO2��I�л�ѧ�������ǹ��ۼ���EΪSi��SiԪ����Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壬ͼ����������Ԫ��ԭ�ӵİ뾶�ɴ�С������Si��C��N��O��H��
�ʴ�Ϊ�� �����ۼ����������ڵڢ�A�壻Si��C��N��O��H��
�����ۼ����������ڵڢ�A�壻Si��C��N��O��H��
��2����Ӧ�ܵĻ�ѧ����ʽΪ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO�����ʴ�Ϊ��2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO������������
��3��FΪNO��BΪO2����F��B�������2��1�����������M����MΪNO2�������²ⶨM��Է�������ʱ���õ���ʵ��ֵ���DZ�����ֵƫ����ԭ����2NO2 N2O4���ʴ�Ϊ��2NO2
N2O4���ʴ�Ϊ��2NO2 N2O4��
N2O4��
��4��FΪNO��ij�ݻ�ΪV L���Թ��г���F���壨��״���£���������������ȫ��ˮ���գ����ݷ�Ӧ����ʽ4NO+2H2O+3O2=4HNO3��֪����ͨ�����������ʵ���Ϊ$\frac{V}{22.4}��\frac{3}{4}$ mol=$\frac{3V}{4��22.4}$mol����ʱ������Һ����ͨ������A��ǡ����ȫ��Ӧ��������Һ�������Һ����Һ��笠�����ˮ�⣬��Һ�����ԣ�������Һ�и�����Ũ���ɴ�С����Ϊc��NO3-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��$\frac{3V}{4��22.4}$��c��NO3-����c��NH4+����c��H+����c��OH-����
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ����������������ۺ�Ӧ����ѧ֪ʶ������ע��������ʵ������Լ�ת����ϵ��Ϊ������Ĺؼ���

| A�� | Al 1s22s22p63s23p1 | B�� | O2-��1s22s22p4 | ||
| C�� | Na��1s22s22p63s1 | D�� | F��1s22s22p5 |
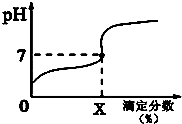 �����£���0.1000mol/L��NaOH��Һ�ζ�20mlͬŨ�ȵ�һԪ����HA���ζ�������ҺpH��X�ı仯������ͼ��ʾ�������к���ЧӦ��������˵������ȷ���ǣ�������
�����£���0.1000mol/L��NaOH��Һ�ζ�20mlͬŨ�ȵ�һԪ����HA���ζ�������ҺpH��X�ı仯������ͼ��ʾ�������к���ЧӦ��������˵������ȷ���ǣ�������| A�� | HA��Һ��ˮϡ�ͺ���Һ��$\frac{c��HA��}{c��{A}^{-}��}$��ֵ��С | |
| B�� | HA�ĵ��볣��KHA=$\frac{1{0}^{-7}X}{100-X}$��xΪ�ζ������� | |
| C�� | ���ζ�����Ϊ100ʱ����Һ��ˮ�ĵ���̶���� | |
| D�� | �ζ���������100ʱ����Һ������Ũ�ȹ�ϵһ����c��Na+����c��A-����c��OH-����c��H+�� |
| A�� | 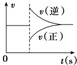 ͼ���Ա�ʾ��ij��ѧƽ����ϵ�ı��¶Ⱥ�Ӧ������ʱ��ı仯 ͼ���Ա�ʾ��ij��ѧƽ����ϵ�ı��¶Ⱥ�Ӧ������ʱ��ı仯 | |
| B�� | 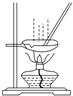 ��ͼ��ʾװ������AlCl3��Һ�Ʊ���ˮAlCl3 ��ͼ��ʾװ������AlCl3��Һ�Ʊ���ˮAlCl3 | |
| C�� |  ͼ���Ա�ʾ��һ������������Һ����εμ�һ��Ũ������������Һʱ����Al��OH��3���������ʵ����仯 ͼ���Ա�ʾ��һ������������Һ����εμ�һ��Ũ������������Һʱ����Al��OH��3���������ʵ����仯 | |
| D�� | 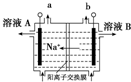 ͼ��ⱥ��ʳ��ˮ��װ���У���ҺA��B����ˮ�������c��H+����A��B ͼ��ⱥ��ʳ��ˮ��װ���У���ҺA��B����ˮ�������c��H+����A��B |
| A�� | �٢� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
| A�� | ��KMnO4��H+����Һ����ױ�����ϩ������ | |
| B�� | ����ˮ���𱽡���ϩ�����Ȼ�̼ | |
| C�� | ��ˮ�����Ҵ����������Ȼ�̼ | |
| D�� | ��NaOH��Һ���𱽷���Һ���ױ��������� |
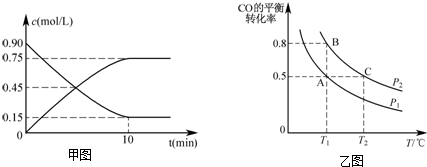
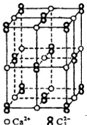 ̼��Ԫ�صĵ��ʼ��仯������һ����Ҫ���ʣ���ش��������⣺
̼��Ԫ�صĵ��ʼ��仯������һ����Ҫ���ʣ���ش��������⣺ ������̼��ԭ��֮�乲�ۼ���c������ţ���
������̼��ԭ��֮�乲�ۼ���c������ţ���