��Ŀ����
����Ŀ�����������Ǻϳ�����ӵ������������������﮵�ԭ�ϡ�ij���������ֳ���������(��Ҫ�ɷ���Fe2O3)Ϊԭ��������ؼ�������������Ĺ����������£�

��1��Ҫ�������������ʣ��˲�ȡ�Ĵ�ʩ��___(������)��
��2��д������ԭ�������м��ǻ��Ϸ�Ӧ����������ԭ��Ӧ�����ӷ���ʽ___��
��3������2�к��е�TiOSO4����ˮ����ˮ�⣬����H2TiO3���÷�Ӧ�Ļ�ѧ����ʽΪ___��
��4������Һ����������õ�һ�ֻ��ʣ��仯ѧʽΪ___����һϵ�в�����ָ___��
��5����֪��298Kʱ��Ksp[Fe(OH)2]=4.0��10-17��������Ũ����1.0��10-5molL-1ʱ���Ӹ���������ȫ���������������У�������ˮ����pH�ķ�ΧΪ___(lg2��0.3)��
��6��������������(FeC2O42H2O)���ȵIJⶨ��ȷ��ȡmg����������������ƿ�У�����һ������ϡ������Һ����������50�棬��cmolL-1KMnO4����Һ�ζ����ﵽ�ζ��յ�ʱ����ȥ����ҺVmL���ζ���Ӧ(δ��ƽ)��FeC2O42H2O+KMnO4+H2SO4��Fe2(SO4)3+CO2+MnSO4+K2SO4+H2O������Ʒ��FeC2O42H2O�Ĵ���Ϊ___%(�ú���m��c��V�Ĵ���ʽ��ʾ)����������������ʧȥ���ֽᾧˮ����õĽ��___(����ƫ������ƫ����������Ӱ����)��
���𰸡����ȡ����衢�۴��۵� 2Fe3++Fe=3Fe2+ TiOSO4+2H2O=H2TiO2��+H2SO4 (NH4)2SO4 ϴ�ӡ����� �� 8.3 ![]() ƫ��
ƫ��
��������
������(��Ҫ�ɷ�ΪFe2O3)�������ܽ⣬���˳�ȥ�������Һ����Ҫ����������������ȣ��������ۻ�ԭ�õ��������������˷��룬��Һ��ͨ�백�����õ��������������������˷��룬��Һ�к�������泥���������������ữ�ϵõ������������پ������ˡ�ϴ�ӡ�����õ������������塣
��Ҫ�������������ʣ��˲�ȡ�Ĵ�ʩ�У����ȡ����衢�۴��۵ȣ��ʴ�Ϊ�����ȡ����衢�۴��۵ȡ�
������ԭ�������з�Ӧ�����ӷ���ʽ���У�Fe + 2Fe3+ = 3Fe2+���ʴ�Ϊ��Fe + 2Fe3+ = 3Fe2+��
��TiOSO4����ˮ����ˮ�⣬����H2TiO3���������������ᣬ��Ӧ����ʽΪ��TiOSO4 + H2O = H2TiO3�� + H2SO4���ʴ�Ϊ��TiOSO4 + H2O = H2TiO3�� + H2SO4��
����Һ�к�������泥���������õ�һ��ũҵ�ϳ��õĻ��ʣ����˺��پ���ϴ�ӡ�����õ������������壬�ʴ�Ϊ��(NH4)2SO4��ϴ�ӡ����
�ɸ������⣬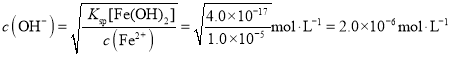 ��pH �� 8.3���ʴ�Ϊ���� 8.3��
��pH �� 8.3���ʴ�Ϊ���� 8.3��
����ƽ��Ӧ����ʽΪ��10FeC2O42H2O + 6KMnO4 + 24H2SO4 = 5Fe2(SO4)3 + 20CO2�� + 6MnSO4 + 3K2SO4 + 24H2O����֪![]() ����
����![]() ����FeC2O42H2O�Ĵ���Ϊ
����FeC2O42H2O�Ĵ���Ϊ![]() ����������������ʧȥ���ֽᾧˮ����õĽ��ƫ�ߣ��ʴ�Ϊ��
����������������ʧȥ���ֽᾧˮ����õĽ��ƫ�ߣ��ʴ�Ϊ��![]() ��ƫ�ߡ�
��ƫ�ߡ�

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ʵ�����У�����ͼ��ʾװ��(β������װ����ȥ)��������ʵ�飬������Һ����ε��뵽���С�ʵ������Ԥ�������һ�µ��ǣ� ��
ѡ�� | ���е����� | ���е����� | Ԥ����е����� |
|
A | Ũ���� | MnO2 ���� | ����������������ɫ���� | |
B | ϡ���� | ̼�������������ƵĻ����Һ | ���������������� | |
C | Ũ���� | ��ɰֽ��ĥ�������� | ������������ɫ���� | |
D | NaOH ��Һ | ��ɰֽ��ĥ�������� | ������������ |
A.AB.BC.CD.D