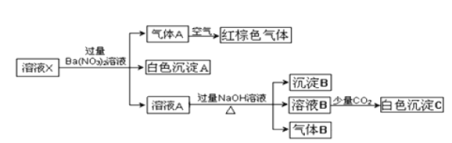
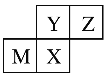��Ŀ����
����Ŀ������˵����ȷ���ǣ�![]()
A.��ʢ�������������Һ���Թ��У��μӼ���������ˮ������������Ȼ��μ�KSCN��Һ�����۲쵽��Һ��ΪѪ��ɫ����˵������������к���![]()
B.����ij��Һ��pH��pH��ֽһ����ˮʪ��һ�������ʵ�����
C.���պ��������������н��У�Ҳ�������������н���
D.ʵ��������֪Ũ�ȵĴ�����Һ�ζ�δ֪Ũ�ȵ�����������Һʱ��ѡ�÷�̪��ָʾ�����ü�����ָʾ��ʱ���������ҪСһ��
���𰸡�D
��������
A���μ�KSCN�������������ټ���ˮ��������Һ��ΪѪ��ɫ�����������ӱ��������������ӣ���˵��ԭ��Һ�к���![]() �����ȼ���ˮ�����KSCN����Һ��Ѫ��ɫ��ֻ��˵������Һ�к���
�����ȼ���ˮ�����KSCN����Һ��Ѫ��ɫ��ֻ��˵������Һ�к���![]() ������˵��ԭ��Һ�к���
������˵��ԭ��Һ�к���![]() ����A����
����A����
B��pH��ֽ�ⶨ��ҺpHʱ������ֽ��ˮʪ���ܻᵼ��ʵ�������ⶨ��ҺΪ������Һ����Խ����Ӱ�죬��B����
C�����ʵ�����Ӧ���������У����ܷ��������������գ���C����
D������ζ�δ֪Ũ�ȵ�����������Һʱ����Ӧ�յ���![]() ���ң�ѡ�÷�̪��ָʾ�����ζ��յ��뷴Ӧ�յ�ӽ����С����ѡ������ָʾ��ʱ�뷴Ӧ�յ���ζ��յ�IJ���������D��ȷ��
���ң�ѡ�÷�̪��ָʾ�����ζ��յ��뷴Ӧ�յ�ӽ����С����ѡ������ָʾ��ʱ�뷴Ӧ�յ���ζ��յ�IJ���������D��ȷ��
��ѡD��

����Ŀ��ˮ��������ԴȪ����ҵ��ѪҺ�����е�������Ҫ�����ú�������ˮ����Ҫ������ˮԴ����Ⱦ��ͨ������ˮ��ֱ�Ӷ������塣Ҳ��ͨ��ʳ�������ũ����Σ����������ش��������⣺
(1)25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ������ӷ���ʽΪ________����ˮ�������c(OH-)=_______mol/L
(2)��ˮ��100��ʱ��pH=6�����¶���1mol/L��NaOH��Һ�У���ˮ������� c(OH-)=_______mol/L��
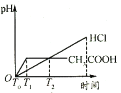
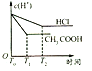
(3)�����Ϊ100mL��pH��Ϊ2��CH3COOH��Һ��һԪ��HX��Һ����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ���ƽ�ⳣ��___(������������С��������������)CH3COOH�ĵ���ƽ�ⳣ����
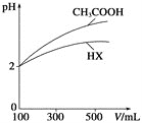
(4)����ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ��������������֪��
��ѧʽ | ����ƽ�ⳣ��(25��) |
HCN | K=4.9��10-10 |
CH3COOH | K==1.8��10-5 |
H2CO3 | K1=4.3��10-7��K2=5.610-11 |
��25��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3Һ��CH3COONa��Һ��������Һ��pH�ɴ�С��˳��Ϊ___��
����NaCN��Һ��ͨ��������CO2��������Ӧ�Ļ�ѧ����ʽΪ______
(5)25��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH=6������Һ�� c(CH3COO-)-c(Na+)=_____(�ȷֵ)mol/L
����Ŀ���ס��ҡ���������Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ����ͼ��ʾ(���ֲ�������ȥ)�����и��������в��ܰ�ͼʾ��ϵת������
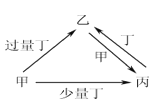
ѡ�� | �� | �� | �� | �� |
A | NaOH | NaHSO3 | Na2SO3 | SO2 |
B | Fe | Fe(NO3)3 | Fe(NO3)2 | HNO3 |
C | C | CO2 | CO | O2 |
D | Al | NaAlO2 | Al(OH)3 | NaOH |
A. A B. B C. C D. D