��Ŀ����
7������A��B��C�����л���������Ϣ���£�| A | B | C |
| ���������࣬��Է�������Ϊ84 ����ȫȼ�յIJ����� n��CO2����n��H2O��=1��1 �۲���ʹ������Ȼ�̼��Һ��ɫ ��һ�ȴ���ֻ�����ֽṹ | ��0.2mol B��ȫȼ�գ�ֻ����35.2g CO2��18g H2O �ھ��ⶨ��0.1mol B������Ϊ7.4g ����Ũ���ᣬ���������£��ɵ�������ʹ��ˮ��ɫ���л����������˳���칹�� | 2�������ĺ��������� �ڷ��ӱ���ģ��Ϊ��  |
��1��A�Ľṹ��ʽΪ
 ����
���� ����
������2��B�ķ���ʽ��C4H10O��B�ڼ��ȣ�Cu��������O2��Ӧ�Ļ�ѧ����ʽΪ��
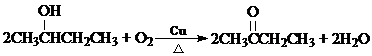 ���䷴Ӧ����Ϊ��������Ӧ��
���䷴Ӧ����Ϊ��������Ӧ����3��XΪB��ͬ����ͬ���칹�壬��X��������2�ֲ�ͬλ�õ���ԭ�ӣ�X��Ũ���ᣬ���������£�������Y��Y��ʹ��ˮ��ɫ��д������Y�Ļ�ѧ����ʽ��
 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$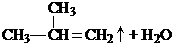 +H2O��
+H2O����4��C��һ�������£���Ӧ���Եõ�һ�����͵ĸ߷������オ����ϣ�PLA����д������PLA�Ļ�ѧ����ʽ��n
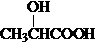 $\stackrel{����}{��}$
$\stackrel{����}{��}$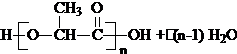 ��
��
���� ��1��������ȫȼ�����ɵĶ�����̼��ˮ�Ĺ�ϵȷʵ���ʽ��Ȼ�������Է�������ȷ�������ʽ��������A�Ļ�ѧ���ʼ�һ�ȴ�������ȷ����ṹ��ʽ��
��2������������Ϣ���������غ㶨��ȷ��B�ķ���ʽ������������������д��B�Ľṹ��ʽ����������ķ�Ӧ����ʽ��
��3���������������γɸ��л���Ľṹ��ʽ��Ȼ����ݴ�����ȥ��Ӧд����Ӧ�Ļ�ѧ����ʽ��
��4������C�ı���ģ��ȷ���京�еĹ����ż��ṹ��ʽ��Ȼ��д��C����ˮ�ⷴӦ����PLA�ķ���ʽ��
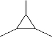
��� �⣺��1����A�������࣬��Է�������Ϊ84������ȫȼ�յIJ�����n��CO2����n��H2O��=1��1����A������C��Hԭ����֮��Ϊ1��2��ͨʽΪCnH2n��������Է�����Ϊ84�ɵã�14n=84����n=6��A�ķ���ʽΪ��C6H12���۲���ʹ������Ȼ�̼��Һ��ɫ��������в�����̼̼˫������һ������1��̼������һ�ȴ���ֻ�����ֽṹ����������к���2�ֵ�ЧH�����������Ľṹ��ʽΪ�� ��
�� ��
��
�ʴ�Ϊ�� ����
���� ����
����
��2����0.2mol B��ȫȼ�գ�ֻ����35.2g CO2��18g H2O����B�����к���C��Hԭ�����ֱ�Ϊ��N��C��=$\frac{\frac{35.2g}{44g/mol}}{0.2mol}$=4��N��H��=$\frac{\frac{18g}{18g/mol}��2}{0.2mol}$=10������C��H����ԭ����Ϊ��12��4+1��10=58���ھ��ⶨ��0.1mol B������Ϊ7.4g����B��Ħ������Ϊ��$\frac{7.4g}{0.1mol}$=74g/mol��������Է�����Ϊ74��58����B������һ��������ԭ�ӣ�������ԭ����Ϊ��$\frac{74-58}{16}$=1����B�ķ���ʽΪ��C4H10O��
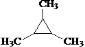
��B��Ũ���ᣬ���������£��ɵ�������ʹ��ˮ��ɫ���л����������˳���칹������B���Ӻ����ǻ��������ǻ�������̼�����ֲ�ͬ��λC����B�Ľṹ��ʽΪ�� ��B�ڼ��ȣ�Cu��������O2��Ӧ�Ļ�ѧ����ʽΪ��
��B�ڼ��ȣ�Cu��������O2��Ӧ�Ļ�ѧ����ʽΪ��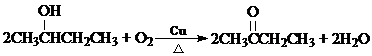
�ʴ�Ϊ��C4H10O��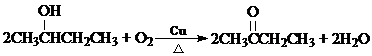
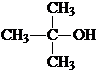
��3��XΪB��ͬ����ͬ���칹�壬��X��������2�ֲ�ͬλ�õ���ԭ�ӣ���X���жԳƽṹ����X��Ũ���ᣬ���������£�������Y��Y��ʹ��ˮ��ɫ����X�����к����ǻ���X�Ľṹ��ʽΪ�� ����Ӧ����Y�Ļ�ѧ����ʽΪ��
����Ӧ����Y�Ļ�ѧ����ʽΪ�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$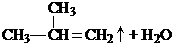 +H2O��
+H2O��
�ʴ�Ϊ�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$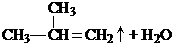 +H2O��
+H2O��
��4����C�������ĺ���������ڷ��ӱ���ģ��Ϊ�� ��˵��C������Ϊ2-�ǻ����ᣬ��ṹ��ʽΪ��
��˵��C������Ϊ2-�ǻ����ᣬ��ṹ��ʽΪ��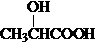 ��C�����к����ǻ����Ȼ����ܹ�����ˮ�ⷴӦ���ɸ߷��ӻ������Ӧ�Ļ�ѧ����ʽΪ��n
��C�����к����ǻ����Ȼ����ܹ�����ˮ�ⷴӦ���ɸ߷��ӻ������Ӧ�Ļ�ѧ����ʽΪ��n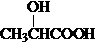 $\stackrel{����}{��}$
$\stackrel{����}{��}$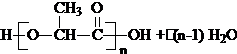 ��
��
�ʴ�Ϊ��n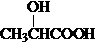 $\stackrel{����}{��}$
$\stackrel{����}{��}$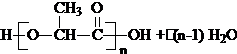 ��
��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע������ȷ���л������ʽ���ṹ��ʽ�ķ�����

| A�� | �����۵�ĸߵͿ��������¹�ϵ��������CA��C�ĵ��ʣ�A��ij�ֵ��� | |
| B�� | A��C��Ԫ�ص�����������Ӧˮ���������A����C | |
| C�� | B��C������������������ǿ�ᷴӦ��������ǿ�Ӧ | |
| D�� | A�ܹ���C�����������л�ԭ���� |
| A�� | ���Ͳ�˿����Ҫ�ɷֶ�����ά�� | |
| B�� | ֲ����������֬������һ�ָ߷��ӻ����� | |
| C�� | ������ϡ����Ĵ��£�����ȫ��ˮ��Ϊ������ | |
| D�� | �����Ѿ��ܷⴢ��ʱ��Խ��������Խ�ã�ԭ��֮һ�Ǵ������������������ζ���� |
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ����ʱ��������NaOH����С�ձ��У��ٷ�����ƽ������ | |
| B�� | �������õĹ���NaOH��������ƿ�У�������ˮ�ܽ� | |
| C�� | ����ʱ�����ˮ�����˿̶��ߣ��ý�ͷ�ι�ֱ���������ಿ�� | |
| D�� | ���ձ����ܽ����NaOH������Һ����ȴ�����º�ת��������ƿ�� |
| A�� | 6��120�� | B�� | 5��108�� | C�� | 4��109��28�� | D�� | 6��109��28�� |
| A�� | ʹ��ˮ�� | B�� | ʹ��֬ˮ�� | C�� | ʹ��������� | D�� | ʹ�����ʱ��� |
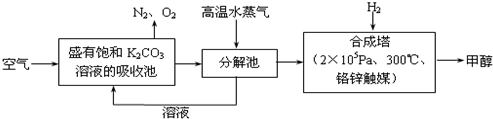
 ��1���������ȡ����Ӧ��ʵ��װ����ͼ��ʾ������AΪ��֧�Թܸ��Ƴɵķ�Ӧ�����������¶˿���һС�ף�����ʯ���ޣ��ټ���������м��
��1���������ȡ����Ӧ��ʵ��װ����ͼ��ʾ������AΪ��֧�Թܸ��Ƴɵķ�Ӧ�����������¶˿���һС�ף�����ʯ���ޣ��ټ���������м��