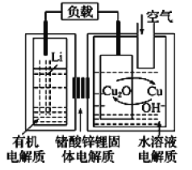
��Ŀ����
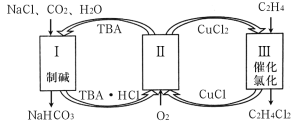
����Ŀ��ʮ�Ŵ����Ҫ�Ի����������ȫ�桢ϵͳ�Ŀɳ�����������ɫ��Դ��ʵʩ�ɳ�����չ����Ҫ;�������������Ҵ�����ȡ��ɫ��Դ�����IJ��ַ�Ӧ��������ͼ��ʾ��

(1)��֪��CO(g) +H2O(g)CO2(g)+H2(g) H1=-41 kJ��mol-1
CH3CH2OH(g)+3H2O(g)2CO2(g)+6H2(g) H2 =+174.1 kJ��mol-1
��ӦI���Ȼ�ѧ����ʽΪ______��
(2)��ӦII�ڽ�����[n(CO) : n(H2O)]��ͬʱ�������Ӧ�� CO ƽ��ת���ʼ���ͼ(�����Ӧ�ķ�Ӧ�¶ȿ�����ͬ��Ҳ���ܲ�ͬ�������Ӧ��������Ӧ��������ͬ)��

��ͼ��A��E�� G�����Ӧ�ķ�Ӧ�¶�TA��TE��TG�Ĺ�ϵ��_____����ԭ���� ______�����¶��£�Ҫ���COƽ��ת���ʣ����˸ı������֮�⣬���ɲ�ȡ�Ĵ�ʩ��______��
����ͼ�п�֪CO��ƽ��ת����������ȡ���Ӧ�¶�֮��Ĺ�ϵ��____��
��A��B �����Ӧ�ķ�Ӧ���ʴ�С��vA_____vB(����<�� ��=������>��)����֪��Ӧ���� v=v��v��= k��x(CO)x(H2O) k��x(CO2) x(H2) ��kΪ��Ӧ���ʳ�����xΪ���ʵ����������ڴﵽƽ��״̬ΪD��ķ�Ӧ�����У���CO��ת���ʸպôﵽ20��ʱ��![]() =_____��
=_____��
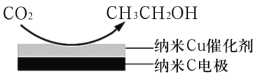
(3)��ӦIII�ڱ���KHCO3���Һ�У������CO2���Ʊ��Ҵ�����ԭ����ͼ��ʾ���������ĵ缫��ӦʽΪ___________��
���𰸡�CH3CH2OH(g)+H2O(g)4H2(g)+2CO(g) H= +256.1 kJ��mol-1 TA=TE=TG KA=KE=KG=1 ��ʱ��ȥ���� �¶���ͬ��������Խ��CO��ƽ��ת����ԽС����������ͬ����Ӧ�¶�Խ�ߣ�CO��ƽ��ת����ԽС �� 36.0 14CO2+12e+9H2O�TCH3CH2OH+12HCO3
��������
(1)��ӦI��ѧ����ʽΪCH3CH2OH(g)+H2O(g)�T4H2(g)+2CO(g)��
��CO(g)+H2O(g)CO2(g)+H2(g)��H1=-41kJ/mol
��CH3CH2OH(g)+3H2O(g)2CO2(g)+6H2(g)��H2=+174.1kJ/mol
���ݸ�˹���ɢ�-����2����CH3CH2OH(g)+H2O(g)�T4H2(g)+2CO(g)����H��
(2)�ٷ�ӦIIΪCO(g)+H2O(g)CO2(g)+H2(g)��H1=-41kJ/mol������Ӧ���ȣ�����A��E��G�����Ӧ��ֵ��Ϸ�Ӧ����ʽ����ƽ�ⳣ��������ƽ�ⳣ���ı仯�����жϣ����ݷ�Ӧ�ص㣬����¶ȡ�Ũ�ȶ�ƽ���ƶ���Ӱ��������COƽ��ת���ʵĴ�ʩ��
����ͼ��֪����CO��ת������ͬʱ���¶��ɵ͵��߶�Ӧ�Ľ�����Ϊ0.5��1��1.5���ɴ˿�ȷ���¶�������ȵĹ�ϵ��
��CO(g)+H2O(g)CO2(g)+H2(g)��H1=-41kJ/mol����ӦΪ���ȷ�Ӧ������ƽ��������У�COת���ʼ�С���ﵽƽ��״̬ΪD��ķ�Ӧ�����У�ƽ�ⳣ��K=![]() ����
����![]() =
=![]() =K��
=K��![]() �����ݷ�Ӧ����ʽ����D��ʱƽ�ⳣ��K������COת���ʸպôﵽ20%ʱ�����ʵ����ʵ�������x������
�����ݷ�Ӧ����ʽ����D��ʱƽ�ⳣ��K������COת���ʸպôﵽ20%ʱ�����ʵ����ʵ�������x������![]() =K��
=K��![]() �м����ֵ��
�м����ֵ��
(3)�����õ��ӷ�����ԭ��Ӧ����ϵ����غ㡢����غ���д�缫��Ӧ����ʽ��
(1)��ӦI��ѧ����ʽΪCH3CH2OH(g)+H2O(g)�T4H2(g)+2CO(g)����CO(g)+H2O(g)CO2(g)+H2(g)��H1=41kJ/mol
��CH3CH2OH(g)+3H2O(g)2CO2(g)+6H2(g)��H2=+174.1kJ/mol
���ݸ�˹���ɢ�����2����CH3CH2OH(g)+H2O(g)�T4H2(g)+2CO(g)����H=+174.1kJ/mol(41kJ/mol)��2=+256.1kJ/mol�����Ȼ�ѧ����ʽΪCH3CH2OH(g)+H2O(g)�T4H2(g)+2CO(g)��H=+256.1kJ/mol��
(2)��ͼ��A����ֵΪ(0.5��66.7)����![]() =0.5��CO��ת����Ϊ66.7%=
=0.5��CO��ת����Ϊ66.7%=![]() �����ݹ�ϵ������ʽ��
�����ݹ�ϵ������ʽ��
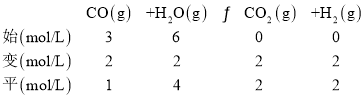
KA=![]() =
=![]() =1��
=1��
ͼ��E����ֵΪ(1��50)����![]() =1��CO��ת����Ϊ50%�����ݹ�ϵ������ʽ��
=1��CO��ת����Ϊ50%�����ݹ�ϵ������ʽ��

ƽ�ⳣ��KE=![]() =
=![]() =1��
=1��
ͼ��G����ֵΪ(1.5��40)����![]() =1.5��CO��ת����Ϊ40%�����ݹ�ϵ������ʽ��
=1.5��CO��ת����Ϊ40%�����ݹ�ϵ������ʽ��
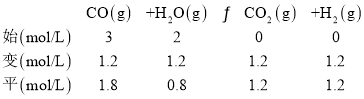
ƽ�ⳣ��KG =![]() =
=![]() =1��
=1��
�������ϼ����֪��KA=KE=KG=1��ƽ�ⳣ�����䣬ƽ�ⳣ�����¶ȵĺ������¶Ȳ��䣬��ƽ�ⳣ�����䣬��ɵ�TA=TE=TG��
CO(g)+H2O(g)CO2(g)+H2(g)��H=41kJmol1��������Ϊ�����������ķ��ȷ�Ӧ�����Ժ��������£�����H2O(g)��Ũ�Ȼ�ʱ�����CO2�Ȳ���������CO��ƽ��ת���ʣ�
����ͼ��֪���¶���ͬ��������Խ��CO��ƽ��ת����ԽС����������ͬ����Ӧ�¶�Խ�ߣ�CO��ƽ��ת����ԽС��
��CO(g)+H2O(g)CO2(g)+H2(g)��H1=41kJ/mol����ӦΪ���ȷ�Ӧ������ƽ��������У�COת���ʼ�С����B���¶ȸߣ���Ӧ���ʿ죬��vA��vB��D����ֵΪ(1��60)����Ӧ����ʽΪ��

D���¶��µ�ƽ�ⳣ��KD��![]() =
=![]() =2.25����Ӧ�ﵽƽ��ʱv��=v������k��x��CO��x��H2O��=k��x��CO2��x��H2��������
=2.25����Ӧ�ﵽƽ��ʱv��=v������k��x��CO��x��H2O��=k��x��CO2��x��H2��������![]() =
=![]() =
=![]() =K=2.25���ڴﵽƽ��״̬ΪD��ķ�Ӧ�����У���COת���ʸպôﵽ20%ʱ����Ӧ����ʽΪ��
=K=2.25���ڴﵽƽ��״̬ΪD��ķ�Ӧ�����У���COת���ʸպôﵽ20%ʱ����Ӧ����ʽΪ��

X��CO��=x��H2O��=![]() =0.4��x��CO2��=x��H2��=
=0.4��x��CO2��=x��H2��=![]() =0.1������
=0.1������![]() =
=![]() =K��
=K��![]() =2.25��
=2.25��![]() =36.0��
=36.0��
(3)�����õ��ӷ�����ԭ��Ӧ���ʵ缫��Ӧ����ʽ14CO2+12e+9H2O�TCH3CH2OH+12HCO3��

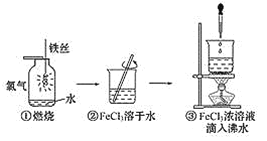
����Ŀ��ij��ѧʵ��С�齫װ��ͭ��Ũ������ƿ����һ��ʱ���ȡ����ƿ�й��壬̽����ɷ֡������Ͽ�֪��Ũ������ͭ��Ӧ��������CuS��Cu2S�����Ƕ�������ˮ��������ϡ���ᡣʵ�����£�
��i��������ˮϴ�ӹ��壬�õ���ɫ��Һ������ʺ�ɫ��
��ii��ȡ������ɫ�������Թ��У���������ϡ���ᣬ��ɫ�������ܽ⣬��Һ��Ϊ��ɫ��������ɫ���ݡ�ȡ�����ϲ���Һ���Թܣ��μ��Ȼ�����Һ��������ɫ������
�ٸ���ʵ�飨i���õ���ɫ��Һ��֪�������к�____________���ѧʽ��
�ڸ���ʵ�飨ii��������_______����������������������ȷ����ɫ������CuS����Cu2S��������__________________________________________________________________________��
д��Cu2S��ϡ���ᷴӦ�Ļ�ѧ����ʽ____________________________________________
��Ϊ�˽�һ��̽����ɫ����ijɷ֣���ʵ�飨i���к�ɫ����ϴ�ӡ���ɣ��ٳ�ȡ48.0g��ɫ�����������ʵ�飬ͨ������O2��ʹӲ�ʲ������к�ɫ�����ַ�Ӧ���۲쵽Fƿ��Ʒ����Һ��ɫ��

ʵ����� | ��Ӧǰ��ɫ��������/g | ��ַ�Ӧ���ɫ��������/g |
I | 48.0 | 48.0 |
�� | 48.0 | 44.0 |
�� | 48.0 | 40.0 |
�����ϱ�ʵ�������Ʋ⣺ʵ��I�к�ɫ����Ļ�ѧʽΪ_____________________________��ʵ�����к�ɫ����ijɷּ�����Ϊ_______________________________________________��