��Ŀ����
8�������������ʣ��ٸɱ� ��NaHCO3���� �۰�ˮ�ܴ������FeCl3��Һ ��ͭ ��Fe��OH��3���� �����ǣ���1���������ڵ���ʵ��Ǣڢܣ�
��2��д��FeCl3�ĵ��뷽��ʽ��FeCl3=Fe3++3Cl-��
��3��д��NaHCO3��aq�� ��ϡ���ᷴӦ�����ӷ���ʽ��HCO3-+H+=CO2��+H2O��
��4��������һ�ֳ����ķ�ɢϵ���ش��������⣮
������е�����ˮ����μ��뱥��FeCl3��Һ�������������Һ�ʺ��ɫ��ֹͣ���ȣ����Ƶ�Fe��OH��3���壬��ȡFe��OH��3���廯ѧ��Ӧ����ʽΪFeCl3+3H2O����ˮ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3HCl��
����Fe��OH��3�����м���Na2SO4������Һ������SO42-���ӣ������ӷ��ţ������ã�ʹ�����γ��˳�����������̽�������ľ۳���
�����ֽ������Һ���õķ������������ЧӦ��
���� ��1����ˮ��Һ�������״̬���ܵ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
��2��FeCl3Ϊǿ����ʣ���ȫ���룻
��3��NaHCO3��aq�� ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��
��4��������е�����ˮ����μ���FeCl3������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȣ����Ƶ�Fe��OH��3���壻
�ڽ���������ʷ����۳���
�۽�����ж����ЧӦ��
��� �⣺��1���ڢ���ˮ��Һ�������״̬���ܵ�����������Ӷ�ʹ��ˮ��Һ������״̬���磬�������ڵ���ʣ��ʴ�Ϊ���ڢܣ�
��2��FeCl3Ϊǿ����ʣ���ȫ���룬���뷽��ʽΪFeCl3�TFe3++3Cl-���ʴ�Ϊ��FeCl3=Fe3++3Cl-��
��3����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ӷ�ӦΪHCO3-+H+=H2O+CO2�����ʴ�Ϊ��HCO3-+H+=CO2��+H2O��
��4����Fe��OH��3������Ʊ����̣�����е�����ˮ����μ���FeCl3������Һ�������������Һ�ʺ��ɫ��FeCl3+3H2O����ˮ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3HCl��ֹͣ���ȣ����Ƶ�Fe��OH��3���壬�ʴ�Ϊ��FeCl3+3H2O����ˮ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3HCl��
����Fe��OH��3�����м���Na2SO4������Һ����������������к����������������ĵ�ɣ����½��巢���˾۳����ʴ�Ϊ��SO42-��
�۽�����ж����ЧӦ���ݴ˿������ֽ������Һ������Ķ����ЧӦ�ǽ������ӶԹ��ߵ�ɢ�������γɵģ��ʴ�Ϊ�������ЧӦ��
���� ���⿼���˵���ʡ��ǵ���ʵĸ�����ӷ�Ӧ����ʽ����д��������Ʊ������ʵȣ��Ƚϻ��������ضԻ���֪ʶ�Ĺ��̣�ע��Ի���֪ʶ���������գ���Ŀ�ѶȲ���ע�����ս�����Ʊ���������������ʼ���������ȷ�������ǵ���ʵ�����

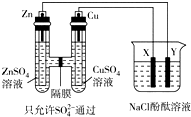 ����ͼ��ʾװ�����ӣ�X��Y��Ϊ���Ե缫�������£���Zn����������3.25gʱ��X����������840mL����״����������ʱ�ձ�����Һ�����Ϊ500mL�����ʱ�ձ�����Һ��pH��������������������ˮ������Һ�ķ�Ӧ����������
����ͼ��ʾװ�����ӣ�X��Y��Ϊ���Ե缫�������£���Zn����������3.25gʱ��X����������840mL����״����������ʱ�ձ�����Һ�����Ϊ500mL�����ʱ�ձ�����Һ��pH��������������������ˮ������Һ�ķ�Ӧ����������| A�� | 11 | B�� | 12 | C�� | 13 | D�� | 14 |
| A�� | ԭ�Ӱ뾶�Ĵ�С˳��r��W����r��Z����r��Y����r��X�� | |
| B�� | Z2+��Ӱ��ˮ�ĵ���ƽ�� | |
| C�� | Y�ļ��⻯������ȶ��Ա�W���� | |
| D�� | Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ |
| A�� | ���ڴ������ð�˾ƥ�ֿ�Ԥ����ð | |
| B�� | �˶�Ա���ڷ�����Ƽ����߳ɼ� | |
| C�� | ע����ù��ǰҪ����Ƥ���������� | |
| D�� | θ�����߷���̼�������к���θ�� |
��1����֪��200�棬101kPaʱ��H2���������������1mol HI�ų�7.45kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪH2��g��+I2��g��$\frac{\underline{\;\;\;200��\;\;\;}}{101kPa}$2HI��g����H=-14.9kJ/mol��
��2��̫���ܵĿ�����������21����һ����Ҫ���⣮���ô��ܽ��ʴ���̫���ܵ�ԭ���ǣ�������̫��������ʹij�����ۻ��������������������ι̻��ͷų���Ӧ��������֪�������ݣ�
| ��� | �� | �۵�/�� | �ۻ���/kJ��mol-1 | �ο��۸�/Ԫ��kg-1 |
| �� | CaCl2•6H2O | 29.0 | 37.3 | 780��850 |
| �� | Na2SO4•10H2O | 32.4 | 77.0 | 800��900 |
| �� | Na2HPO4•12H2O | 36.1 | 100.1 | 1800��2400 |
| �� | Na2SiO3•5H2O | 52.5 | 49.7 | 1400��1800 |
��3�����Ӿ��徧���ܵĶ�������̬�����γ�1mol���Ӿ����ͷŵ�������ͨ��ȡ��ֵ������֪��
2Na��s��+Cl2��g���T2NaCl��s����H=-821.8kJ��mol-1
2Cl��g�TCl��g����H=-239.4kJ��mol-1
Na��s���TNa��g����H=+108.8kJ��mol-1
Na��g���TNa+��g��+e-��H=+496.0kJ��mol-1
Cl��g��+e-�TCl-��g����H=-348.7kJ��mol-1
��NaCl�ľ�����Ϊ786.7kJ/mol��
| A�� | ���������ƾ��ƣ������ľƾ�������ȼ��ʱ��Ӧ������ʪĨ���˸� | |
| B�� | ������Ũ���ὦ��Ƥ���ϣ�Ӧ���ò�Ĩȥ�����ô���ˮ��ϴ�����Ϳ��̼��������Һ | |
| C�� | ��ȡ����ˮʱ��Ϊ��ֹ��ƿ�ڲ�����������Ӧ������ƿ�м��뼸Ƭ���Ƭ | |
| D�� | ʵ������ȡ������Ϻ�Ӧֹͣ���ȣ���ȡ������ƿ�����ȡ�������� |