��Ŀ����
16��ijͬѧ�����һ���״���CH3OH��ȼ�ϵ�أ����øõ�ص��200mLһ��Ũ��NaCl��CuSO4�����Һ����װ����ͼ1��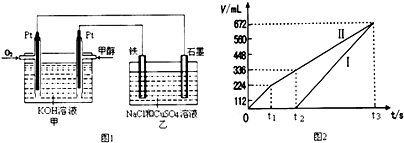
��1��25�棬1.01��105Paʱ16gҺ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�362.9kJ�������˷�Ӧ���Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2=CO2��g��+2H2O��l����H=-725.8kJ/mol��
��2��д������ͨ��״���һ���ĵ缫��ӦʽCH3OH-6e-+8OH-=CO32-+6H2O��
��3�����缫�Ϸ�Ӧ������Ϊ�����к�ɫ�������������������ݲ�����ʯī�缫�ϲ�����������Cl2��O2��
��4������������������������������ʱ��仯�Ĺ�ϵ��ͼ2��ʾ����������ѻ���ɱ�״���µ������ԭ�����Һ��CuSO4�����ʵ���Ũ��0.1mol•L-1����������Һ������䣩
���� ��1��16g Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�369.2kJ����������1mol Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�725.8kJ���������ݴ���д��
��2���״�����������Ӧ���ڼ�������������̼���������ˮ��
��3�����ͨ��״���Ϊ�������������缫Ϊ������ʯī�缫Ϊ��������ʼCl-�������ŵ���������������Cu2+�ŵ�����Cu����ͼ3�Т��ʾ�������������壬���ʾ�������������壬����t1ǰ�缫��ӦʽΪ��2Cl--4e-=Cl2����t1��t3�缫��ӦʽΪ��4OH--4e-=O2��+2H2O������t2ǰ�缫��ӦʽΪ2Cu2++4e-=2Cu��t2���缫��ӦʽΪ4H++4e-=2H2����
��4������t1��t2 ������������������������ϵ�ʧ�����غ����CuSO4�����ʵ���Ũ�ȣ�
��� �⣺��1��16g Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�369.2kJ����������1mol Һ̬�״���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�725.8kJ�����������Ȼ�ѧ����ʽΪ��CH3OH��l��+$\frac{3}{2}$O2=CO2��g��+2H2O��l����H=-725.8kJ/mol��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2=CO2��g��+2H2O��l����H=-725.8kJ/mol��
��2����ȼ�ϵ�ص��У���������ȼ�Ϸ���ʧ���ӵ�������Ӧ���ڼ��Ի����£��״�ʧ���ӵĹ���Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
��3�����ͨ��״���Ϊ�������������缫Ϊ������ʯī�缫Ϊ��������ʼCl-�������ŵ���������������Cu2+�ŵ�����Cu����ͼ3�Т��ʾ�������������壬���ʾ�������������壬����t1ǰ�缫��ӦʽΪ��2Cl--4e-=Cl2����t1��t3�缫��ӦʽΪ��4OH--4e-=O2��+2H2O������t2ǰ�缫��ӦʽΪ2Cu2++4e-=2Cu��t2���缫��ӦʽΪ4H++4e-=2H2�����������缫�Ϸ�Ӧ������Ϊ�����к�ɫ�������������������ݲ�����ʯī�缫�ϲ�����������Cl2��O2���ʴ�Ϊ�������ݲ�����Cl2��O2��
��4��ʯīΪ���������缫Ϊ��������ʼCl-�������ŵ���������������Cu2+�ŵ�����Cu����ͼ3�Т��ʾ�������������壬���ʾ�������������壬t1ǰ�缫��ӦʽΪ������2Cl--4e-=Cl2����t2���缫��ӦʽΪ������4OH--4e-=O2��+2H2O��
��ͼ��֪����������Ϊ224mL������2Cl--2e-=Cl2����֪��n��NaCl��=$\frac{0.224L}{22.4L/mol}$��2=0.02mol����t2ʱ��������Ϊ112mL��n��O2��=$\frac{0.112L}{22.4L/mol}$=0.005mol����ת�Ƶ���Ϊ0.02mol+0.005mol��4=0.04mol��
���ݵ����غ㼰Cu2++2e-=Cu��֪��n��CuSO4��=$\frac{0.04mol}{2}$=0.02mol������c��CuSO4��=$\frac{0.02mol}{0.2L}$=0.1mol/L��
�ʴ�Ϊ��0.1��
���� ���⿼����ԭ������ȷ�����ĵ缫��Ӧ��ͼͼ��Ķ�Ӧ��ϵ�ǽ����Ĺؼ�������ץס��������ת����ȼ��㣬��Ŀ�Ѷ��еȣ�

 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�| A�� |  | B�� |  | C�� |  | D�� |  |
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c1 | 0.2 | pH=7 |
| �� | 0.1 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��1���������������ʵ���������Ӽ�����������������a�������Һ��pH����˵��HA��ǿ�ỹ������a=7��HAΪǿ���a��7��HAΪ���ᣮ
��2���������������ʵ�����������������������c1�Ƿ�һ������0.2mol/L����ǡ����������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ��C��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3���ӱ���ʵ����������HA�����ᣨ�ǿ�������������û����Һ��c��A-��+c��HA��=c��Na+����0.05mol/L��
| A�� | Na2O2����������ߵĹ����� | B�� | ��ҵ�ϵ������״̬Al2O3�Ʊ�Al | ||
| C�� | ��ҵ�����úϳɰ�ʵ���˹��̵� | D�� | ʵ������NH4Cl ��Ca��OH��2�Ʊ�NH3 |
| A�� | D��T��Ϊͬλ�� | B�� | C2H6��CH3CH2CH2CH3��Ϊͬϵ�� | ||
| C�� | C��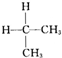 �� ��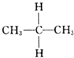 ��Ϊͬ���칹�� ��Ϊͬ���칹�� | D�� | O2��O3��Ϊͬ�������� |
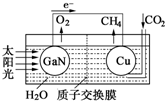
| A�� | ��װ��Ϊԭ��أ���ͭΪ���� | |
| B�� | ��ع���ʱ��H+��Cu�缫�ƶ� | |
| C�� | GaN�缫����ĵ缫��ӦʽΪ��2H2O-4e-�TO2+4H+ | |
| D�� | ��ӦCO2+2H2O�TCH4+2O2��ÿ����1mol CO2ת��4mol e- |
| A�� | �����ŷŵ�����������γ����ꡢ�����⻯ѧ��������в��������� | |
| B�� | ʳ�õع��Ͷ�����Σ�����ع��Ϳ������Ʒ��� | |
| C�� | ����ˮ�������Al��OH��3���壬������ˮ��ɱ������ | |
| D�� | ���ǿ�����Ԫ���������ڹ���Ԫ����Ѱ�����������Ĵ��� |