��Ŀ����
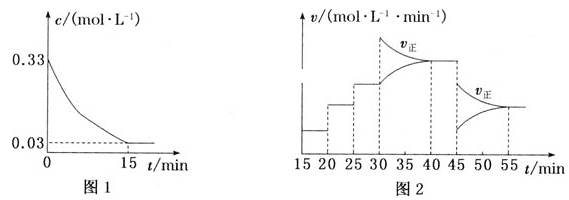
����Ŀ����ͼ��ʾ��ijͬѧ�����һ������(CH3OCH3)ȼ�ϵ�ز�̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ������װ����XΪ�����ӽ���Ĥ������Ҫ��ش�����������⣺
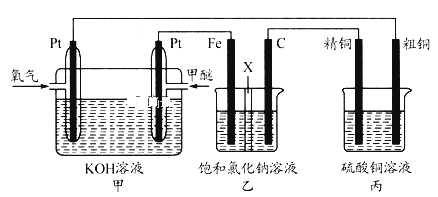
��1��д�����и����ĵ缫��Ӧʽ__��
��2����Ӧһ��ʱ�����װ��������NaOH��Ҫ��___(��������������ʯī����)����
��3�������ͭ�к���п���������ʣ���װ���������ϵ缫��ӦʽΪ___����Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ�___(��������������С������������)��
��4�����ڱ�״���£���2.24L�����μӷ�Ӧ������װ�������缫�����ɵ������ڱ�״���µ����Ϊ___��
���𰸡�CH3OCH3-12e-+16OH-�T2CO32-+11H2O ���� Zn-2e��=Zn2+��Cu-2e-=Cu2+ ��С 4.48L
��������
�������⣬��Ϊ����(CH3OCH3)ȼ�ϵ�أ��Һͱ������ڵ��ء���װ���У�ͨO2һ��Ϊ��������缫����ʽΪ��3O2+6H2O+12e-=12OH-��ͨ�����һ��Ϊ��������缫��ӦΪCH3OCH3-12e-+16OH-�T2CO32-+11H2O���ܵķ�Ӧ����ʽΪCH3OCH3+3O2+4OH-=2CO32-+5H2O����װ���У�FeΪ������CΪ��������������ʽΪ��2H2O+2e-=2OH-+H2������������ʽΪ��2Cl--2e-=Cl2������װ���У���ͭΪ��������Ҫ�ĵ缫��ӦΪCu-2e-=Cu2+����ͭΪ�������缫��ӦΪCu2++2e-=Cu��
��1����Ϊ����(CH3OCH3)ȼ�ϵ�أ��为���ĵ缫��ӦΪ��CH3OCH3-12e-+16OH-�T2CO32-+11H2O��
��2����װ���ǵ�ⱥ��ʳ��ˮ��װ�ã���NaOH�������ϲ����������������װ�õĸ�����������
��3����װ��Ϊ��ͭ��װ�ã����д�ͭΪ���������ڴ�ͭ�к���п���������ʣ���п�Ļ����Ա�ͭǿ�����������ĵ缫��Ӧ�У�Zn-2e��=Zn2+��Cu-2e-=Cu2+����Ӧһ��ʱ������������ŵ��Cu2+�������ŵ�����Zn2+��Cu2+��ͬȡ����������Һ��CuSO4��Ũ�ȼ�С��
��4������£�n(O2)=![]() =
=![]() =0.1mol�����·�е���ת��0.4mol������װ��������������H2�����ʵ���Ϊ0.2mol�������µ����Ϊ0.2mol��22.4L/mol=4.48L��
=0.1mol�����·�е���ת��0.4mol������װ��������������H2�����ʵ���Ϊ0.2mol�������µ����Ϊ0.2mol��22.4L/mol=4.48L��

 ��У����ϵ�д�
��У����ϵ�д�