题目内容
已知实验:①0.1 mol·L-1 AgNO3溶液和0.1 mol·L-1 NaCI溶液等体积混合得到浊液a,过滤得到滤液b和白色沉淀;② 向滤液b中滴加0.1 mol·L-1 KI溶液,出现浑浊;③ 向沉淀c中滴加0.1 mol·L-1 KI溶液,沉淀变为黄色。下列分析不正确的是( )
A.浊液a中存在沉淀溶解平衡:AgCl(s) Ag+(aq)+Cl—(aq) Ag+(aq)+Cl—(aq) |
| B.滤液b中不含有Ag+ |
| C.③中颜色变化说明AgCl 转化为AgI |
| D.实验可以证明AgI比AgCl更难溶 |
B
解析试题分析:A、根据浊液a中含有AgCl,存在沉淀溶解平衡:AgCl(s)?Ag+(aq)+Cl-(aq),正确;B、滤液为AgCl的饱和溶液,也存在沉淀的溶解平衡,即存在Ag+,错误;C、向AgCl中滴加0.1mol?L-1KI溶液,白色AgCl沉淀变为黄色AgI沉淀,正确;D、向AgCl中滴加0.1mol?L-1KI溶液,白色AgCl沉淀变为黄色AgI沉淀,实验证明AgI比AgCl更难溶,正确。
考点:本题考查沉淀溶解平衡及沉淀转化的本质。

 53随堂测系列答案
53随堂测系列答案取浓度相同的NaOH和HCl溶液,以3∶2体积比相混合,所得溶液的pH等于12,则原溶液的浓度为
| A.0.01 mol·L-1 | B.0.017 mol·L-1 | C.0.05 mol·L-1 | D.0.50 mol·L-1 |
下列溶液中有关微粒的物质的量浓度关系正确的是
| A.物质的量浓度相等的① NH4HSO4溶液、② NH4HCO3溶液、③ NH4Cl溶液中,pH的大小关系:②>①>③ |
| B.常温下,将CH3COONa溶液和稀盐酸混合至溶液pH=7: c(Na+)>c(CH3COO-)>c(Cl-)=c(CH3COOH)>c(H+)=c(OH-) |
| C.常温下,pH=6的NaHSO3溶液中:c(SO32-)-c(H2SO3)=9.9×10-7 mol·L-1 |
| D.物质的量浓度之比为1∶2的NaClO、NaHCO3混合溶液中: |
对于常温下PH为2的盐酸,叙述正确的是
| A.c(H+) = c(Cl—) + c(OH—) |
| B.由H2O电离出的c(H+) =" 1.0" ×10—12 mol·L—1 |
| C.与等体积PH=12的氨水混合后所得溶液显酸性 |
| D.与等体积0.01 mol·L—1乙酸钠溶液混合后所得溶液中:c(Cl—) = c(CH3COO—) |
常温下,向一定体积的1mol.L-1的醋酸溶液中逐滴加入等浓度的氢氧化钠溶液,溶液中pOH和pH的变化如图所示,已知该条件下醋酸的电离程度为0.4%,则下列说法不正确的是
| A.a为7 |
| B.Q点的水的电离程度在整条曲线中最大 |
| C.M和Q之间(不含Q)的溶液离子浓度排序都满足c(CH3COO-)>c(Na+) |
| D.三点所代表的溶液导电能力,N最大,M最小 |
AgCl(s)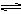 Ag+(aq) + Cl-(aq),平衡时,Ksp =C(Ag+ )·C(Cl-) ,过量氯化银分别投入①100 mL水 ②24 mL 0.1 mol·L-1NaCl ③10 mL 0.1 mol·L-1MgCl2 ④30 mL 0.1 mol·L-1AgNO3溶液中,溶液中C(Ag+)由大到小顺序为
Ag+(aq) + Cl-(aq),平衡时,Ksp =C(Ag+ )·C(Cl-) ,过量氯化银分别投入①100 mL水 ②24 mL 0.1 mol·L-1NaCl ③10 mL 0.1 mol·L-1MgCl2 ④30 mL 0.1 mol·L-1AgNO3溶液中,溶液中C(Ag+)由大到小顺序为
| A.③①②④ | B.④①②③ | C.①②③④ | D.④③②① |
根据下列实验内容得出的结论正确的是
| | 实验操作 | 解释或结论 |
| A |  沉淀中滴入浓KCI溶液有白色沉淀出现 沉淀中滴入浓KCI溶液有白色沉淀出现 |  比 比 更难溶 更难溶 |
| B | 某物质的水溶液能使红色石蕊试纸变蓝 | 该物质一定是氨气 |
| C | 滴加氯水和 振荡,静置,下层溶液为紫色 振荡,静置,下层溶液为紫色 | 原溶液中有 |
| D | 用洁净的铂丝蘸取溶液进行焰色反应,火焰呈黄色 | 含有Na单质 |
室温下,下列溶液等体积混和,所得溶液pH的一定大于7的是( )
| A.0.1mol.l-1盐酸溶液和0.1mol.l-1氢氧化钠溶液 |
| B.0.1mol.l-1盐酸溶液和0.1mol.l-1氢氧化钡溶液 |
| C.pH =4醋酸溶液和pH =10氢氧化钠溶液 |
| D.pH =4盐酸溶液和pH =10氢氧化钡溶液 |
下列各溶液中,微粒的物质的量浓度关系正确的是( )
| A.0.1 mol/L Na2CO3溶液:c(OH-)=c(HCO3-)+c(H+)+2c(H2CO3) |
| B.0.1 mol/L NH4Cl溶液:c(NH4+ )=c(Cl-) |
| C.向醋酸钠溶液中加入适量醋酸,得到的酸性混合溶液:c(Na+)>c(CH3COO-)>c(H+)>c(OH-) |
| D.向硝酸钠溶液中滴加稀盐酸得到的pH=5的混合溶液:c(Na+)>c(NO3-) |