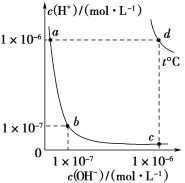
��Ŀ����
����Ŀ����һ����NO2��N2O4�Ļ������ͨ�����Ϊ1 L�ĺ����ܱ������У�������Ũ����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��

(1)����÷�Ӧ��ƽ�ⳣ��K��____����Ӧ���е�20 minʱ�����������ڳ���һ����NO2,10 min��ﵽ�µ�ƽ�⣬��ʱ���c(NO2)��0.9 mol��L��1��
(2)��һ��ƽ��ʱ���������NO2���������Ϊw1���ﵽ��ƽ�����������NO2���������Ϊw2����w1___w2(����>��������������<��)��
(3)������ͼ�л���20 min������ʵ�Ũ����ʱ��仯������___ (�����ϱ�������X������Y��)��

���𰸡�0.9 > 
��������
��ͼ1��֪��X��Y��Ũ�ȱ仯��֮��Ϊ2��1����XΪNO2��YΪN2O4��������Ӧ��N2O4��g��![]() 2NO2��g����
2NO2��g����
��1��ƽ��ʱc��NO2��=0.6mol��L��1��c��N2O4��=0.4mol��L��1������K=![]() ���㣻
���㣻
��2�����º����£��ٳ���һ����NO2����ЧΪ����ѹǿ��ƽ�������ƶ���
��3��20minʱ˲��c��NO2������c��N2O4�����䣬����ƽ�����淴Ӧ�����ƶ���c��NO2����С��c��N2O4������10min��ﵽ�µ�ƽ�⣬��ʱ���c��NO2��=0.9mol��L��1������ƽ�ⳣ������ƽ��ʱc��N2O4�����ݴ���ͼ��
��1��ƽ��ʱc��NO2��=0.6mol��L��1��c��N2O4��=0.4mol��L��1������K=![]() =
=![]() =0.9��
=0.9��
��2�����º����£��ٳ���һ����NO2����ЧΪ����ѹǿ��ƽ�������ƶ�����ƽ�����������NO2�����������С����W1��W2��
��3��20minʱ˲��c��NO2������c��N2O4�����䣬����ƽ�����淴Ӧ�����ƶ���c��NO2����С��c��N2O4������10min��ﵽ�µ�ƽ�⣬��ʱ���c��NO2��=0.9mol��L��1������K=![]() ==0.9����ƽ��ʱc��N2O4��=
==0.9����ƽ��ʱc��N2O4��=![]() mol��L��1=0.9mol��L��1����XΪNO2��YΪN2O4��20min������ʵ�Ũ����ʱ��仯������Ϊ��
mol��L��1=0.9mol��L��1����XΪNO2��YΪN2O4��20min������ʵ�Ũ����ʱ��仯������Ϊ��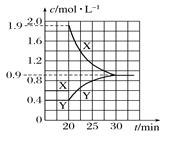 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ�鷽���У����Դﵽʵ��Ŀ�ĵ��ǣ�������
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | �������������Ƿ���� | �Ƚ�����������Ʒ����ˮ�����Һ��Ȼ���������ϡ�����ữ���ټ��� |
B | ��ȥ���л��еı��� | ������������ˮ����ַ�Ӧ����ˣ���ȥ���� |
C | ��ȥNaCl�����л��� | �Ƚ���������ˮ�����Һ��Ȼ�������ᾧ�����ȹ�����ȥ��Һ |
D | ���� | �� |
A.AB.BC.CD.D
����Ŀ������ʵ�����������ͽ��۾���ȷ����
ѡ�� | ���� | ���� | ���� |
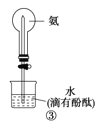
A | ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ�
| ��ֽ����ɫ |
|
B | �������� | �²�Һ����ɫ��dz |
|
C | ���������� | �۲쵽��ɫ��Ȫ |
|
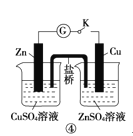
D | ���պϿ���K���γ�ԭ��� | Zn�����к�ɫ�������� | п�Ľ����Ա�ͭǿ |
A.AB.BC.CD.D