��Ŀ����
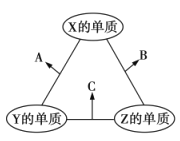
����Ŀ��ijУʵ��С���ͬѧ������ͼ��ʾʵ��װ��̽����������ͭ�ķ�Ӧ��ͼ�мг֡��̶�װ�þ���ȥ����
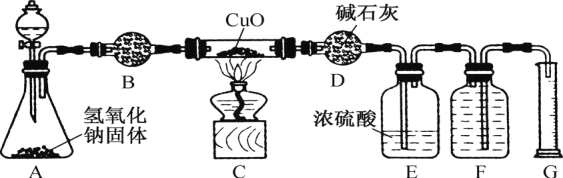
��1����ͼ�����װ����������__________________����װ��ҩƷ��
��2��A�з�Һ©����Ӧװ_________________ ��B�й����Լ�Ϊ__________________��
��3����Һ©�������������Լ�����ȼC���ľƾ��ƣ�һ��ʱ��۲쵽C������ͭȫ��ת���ɹ����ĺ�ɫ���壬F�м���ƿ���ռ���һ����ɫ��̬���ʡ�д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��______��
��4��E��Ũ�����������__________________��
��5����ƽ���ƶ�ԭ������A�в��������ԭ��__________________________ ��������ص����ӷ���ʽ�ͱ�Ҫ������������
��6����֪Cu2OҲ�Ǻ�ɫ�ġ��±�Ϊʵ��ǰ��Cװ�õ�������ͨ�������֪��Ӧ��IJ�����______ (����������������������ȷ����)Cu2O��
�ղ����� | ʵ��ǰ(��Ʒ+������) | ʵ���(��Ʒ+������) |
59.60 g | 65.60g | 64.64g |
���𰸡���������� Ũ��ˮ ��ʯ�� 2NH3+3CuO![]() 3Cu+N2+3H2O ����δ��Ӧ��NH3 ����������������ˮ���ȣ����Լ�С�������ܽ�ȣ�ͬʱ����������������ӣ�����������Ũ������ʹ��NH3 + H2O
3Cu+N2+3H2O ����δ��Ӧ��NH3 ����������������ˮ���ȣ����Լ�С�������ܽ�ȣ�ͬʱ����������������ӣ�����������Ũ������ʹ��NH3 + H2O ![]() NH3��H2O
NH3��H2O![]() NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ� ��
NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ� ��
��������
AΪ�����ķ���װ�ã�BΪ����װ�ã�CΪ����������ͭ�ķ�Ӧװ�ã�E��Ũ�����������δ��Ӧ�İ�����F��GΪ�ռ����ɵ�������������������װ�ã�������ʵ����ʷ������
(1)ʵ����������ɰ��������������ķ�Ӧ�������Ӻ�װ�ú�Ӧ���ȼ��װ�õ������ԣ��ʴ�Ϊ�����װ�õ������ԣ�
(2)����ͼʾ��AΪ�Ʊ�������װ�ã�A�з�Һ©����ӦװŨ��ˮ����εμӵ����������������Ʊ�����������Ϊ�������壬Ӧѡ����Ը����������ѡ�ü�ʯ�Ҹ��ﰱ�����ʴ�Ϊ��Ũ��ˮ����ʯ�ң�
(3)��Һ©�������������Լ�����ȼC���ľƾ��ƣ�һ��ʱ��۲쵽C������ͭȫ��ת���ɹ����ĺ�ɫ���壬F�м���ƿ���ռ���һ����ɫ��̬���ʣ�˵������������ͭ��Ӧ����ͭ�͵�����ˮ����Ӧ�Ļ�ѧ����ʽ��2NH3+3CuO![]() 3Cu+N2+3H2O���ʴ�Ϊ��2NH3+3CuO
3Cu+N2+3H2O���ʴ�Ϊ��2NH3+3CuO![]() 3Cu+N2+3H2O��
3Cu+N2+3H2O��
(4)E��Ũ�����������δ��Ӧ�İ������ʴ�Ϊ������δ��Ӧ�İ�����
(5)����������������ˮ���ȣ����Լ�С�������ܽ�ȣ�ͬʱ����������������ӣ�����������Ũ������ʹ��NH3 + H2O ![]() NH3��H2O
NH3��H2O![]() NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ����ʴ�Ϊ������������������ˮ���ȣ����Լ�С�������ܽ�ȣ�ͬʱ����������������ӣ�����������Ũ������ʹ��NH3 + H2O
NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ����ʴ�Ϊ������������������ˮ���ȣ����Լ�С�������ܽ�ȣ�ͬʱ����������������ӣ�����������Ũ������ʹ��NH3 + H2O ![]() NH3��H2O
NH3��H2O![]() NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ���
NH4+��OH��ƽ�������ƶ�����������Ũ�ȣ�ʹ�����������ӷ��ݳ���
(6)�ɱ������ݿ�֪��ʵ��ǰ����ͭ������=65.60g-59.60g=6g��ʵ����������������Ϊ��65.60g-64.64g=0.96g��
���ݷ���ʽ������ͭ��ȫ��Ӧ����ͭ���ĵ�����Ϊm����
2NH3+3CuO![]() 3Cu+N2+3H2O ��m
3Cu+N2+3H2O ��m
240 48
6g m
![]() =
=![]() ����ã�m=1.2g��ʵ����������Ϊ0.96g��С��1.2g��˵����������ͭ���ɣ��ʴ�Ϊ���С�
����ã�m=1.2g��ʵ����������Ϊ0.96g��С��1.2g��˵����������ͭ���ɣ��ʴ�Ϊ���С�

����Ŀ�����м������ʵ��۵����������ʾ��
A�� | B�� | C�� | D�� |
���ʯ��3550�� |
|
|
|
�辧�壺1410�� |
|
|
|
���壺2300�� |
|
|
|
�������裺1723�� |
|
|
|
��ش��������⣺
��1��A������______���壬���ۻ�ʱ�˷����������������______��
��2��B�龧�干ͬ������������______������ţ���
A���н������� B���е����� C���е����� D������չ��
��3��C����������������______����ȶ�����______��
��4��D�龧����ܾ��е�������______������ţ���
A��Ӳ��С B��ˮ��Һ�ܵ��� C�������ܵ��� D������״̬���ܵ���