��Ŀ����
����Ŀ����̼������Ҫ��ѧ�ɷ�ΪCeFCO3��������ȡ����ϡ��Ԫ�ص���Ҫ����ԭ�ϣ���������(CeO2) ��һ����Ҫ��ϡ��������Է�̼���Ϊԭ���Ʊ�CeO2��һ�ֹ�������������
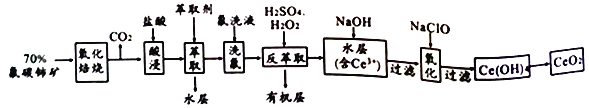
��֪����Ce 4+������F ��ϳ�[CeFx](4-x)+,Ҳ����SO42-��ϳ�[CeSO4]2+��
����������ϵ��Ce4+ �ܱ���ȡ��[(HA)2]��ȡ����Ce3+���ܡ�
�ش��������⣺
��1�� ��������������Ŀ����______________��
��2����������л������������ɫ���塢��д��CeO2�����ᷴӦ�����ӷ���ʽ___________________��Ϊ�������������Ⱦ�������һ�ֽ������________________________��
��3������ȡ��ʱ���ڷ�Ӧ��Ce4++n(HA)2![]() Ce+(H2a-4A2a)+4H+��ʵ��������ȡʱ�õ�����Ҫ��������Ϊ______________����ȱ�� ��ϴ�����������ò�Ʒ��������_________(����ƫ��������ƫС���� ��������)��
Ce+(H2a-4A2a)+4H+��ʵ��������ȡʱ�õ�����Ҫ��������Ϊ______________����ȱ�� ��ϴ�����������ò�Ʒ��������_________(����ƫ��������ƫС���� ��������)��
��4�� ������ȡ���У���ϡ�����H2O2������__________________(�����ӷ���ʽ��ʾ)��
��5��ȡ���������еõ���CeO2��Ʒ0.500g���������ܽ����0.1000mol��L-1FeSO4����Һ�ζ����յ�ʱ(�汻��ԭΪCe3+���������ʾ����μӷ�Ӧ)��25.00mL����Һ���ò�Ʒ��CeO2����������Ϊ________��
���𰸡� ��+3����������+4��(��CeFCO3������CeO2) 2CeO2+8H++2Cl-=2Ce3++Cl2��+4H2O ����H2SO4��� ��Һ©�� ƫС Ce4++H2O2=2Ce3++O2��+2H+ 86.0%��0.86
�������������������1�����������ա����Խ���+3����������+4������2��������������ᱻCeO2����Ϊ����ɫ������CeO2����ԭΪCe3+��Ϊ����������ԭ��Ӧ���������Ը�����������3������ȡ��ʱҪ��Һ������Ce4+����F-��ϳ�[CeFx](4-x)+��������4����������ϵ��Ce4+�ܱ���ȡ��[(HA)2]��ȡ����Ce3+������������ȡ���У���ϡ�����H2O2���Խ�Ce4+��ԭΪCe3+����5�����ݵ����غ�FeSO4��CeO2�Ĺ�ϵʽ��FeSO4![]() CeO2�����ù�ϵʽ������
CeO2�����ù�ϵʽ������
��������1�����������ա����Խ�CeFCO3��+3����������CeO2��+4��������2��������������ᱻCeO2����Ϊ����ɫ������CeO2����ԭΪCe3+�����ӷ���ʽ��2CeO2+8H++2Cl-=2Ce3++Cl2��+4H2O ��Ϊ����������ԭ��Ӧ���������Ը���H2SO4�������3������ȡ��ʱҪ��Һ���õ�����Ҫ���������Ƿ�Һ©����Ce4+����F-��ϳ�[CeFx](4-x)+����ȱ����ϴ�����������ò�Ʒ��������ƫС����4����������ϵ��Ce4+�ܱ���ȡ��[(HA)2]��ȡ����Ce3+������������ȡ���У���ϡ�����H2O2���Խ�Ce4+��ԭΪCe3+����Ӧ���ӷ���ʽ��Ce4++H2O2=2Ce3++O2��+2H+��
��5����CeO2��������xg
FeSO4 ![]() CeO2 ��
CeO2 ��
1mol 172g
0.025L![]() 0.1mol��L-1 xg
0.1mol��L-1 xg
![]()
X=0.43g���ò�Ʒ��CeO2����������Ϊ![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����֪a��b��c��d��e��f�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������a��cԭ�ӵ�L����2��δ�ɶԵ��ӣ�d��eͬ����,d�Ķ�����������c�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��f3+����M��3d�������Ϊ�����״̬�������������Ϣ���ش���������(����ʱ��������Ӧ��Ԫ�ط��ű�ʾ)��
��1������f3+���ӵļ۲�����Ų�ͼ__________��bH3������bԭ�ӵļ۲���ӶԹ���Ϊ__________��
��2��д��һ����ab-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ_________��ab-��aԭ�ӵ��ӻ���ʽΪ_______��
��3��f��m(������Ϊ25) ��Ԫ�صIJ��ֵ��������������±���
Ԫ�� | m | f | |
����(kJ��mol-1) | I1 | 717 | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
�Ƚ���Ԫ�ص�I2��I3��֪����̬m2+��ʧȥһ�����ӱ���̬f2+��ʧȥһ�������ѣ�ԭ����____________��
��4����֪e��̼�������ȷֽ��¶ȱ�d�ĸߣ���ԭ����___________________��
��5����֪CaF2���峣�������ۼ����侧���ṹ��ͼ��ʾ��
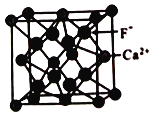
�谢���ӵ�������ֵΪNA ��Fԭ�Ӻ�Caԭ��֮��ľ���Ϊapm���ھ�����Խ��ߵ�1/4��3/4����ֱ��и�F-����Ca2+����λ����_______��������ܶ�Ϊ_______��