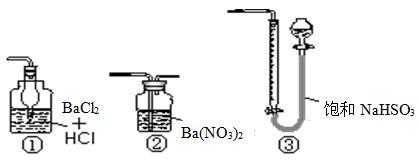
��Ŀ����
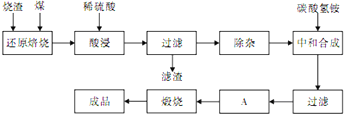
��10�֣���Ϣʱ�������Ĵ������������Ի�������������в��ij�о���ѧϰС�齫һ����������·������õ���Cu��Fe������Au��Pt�Ƚ����Ļ����������������Ʊ��������壨CuSO4?5H2O����
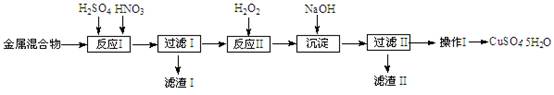
��֪������������������������ʽ����ʱ��Һ��pH���±���
��1�����������Ҫ�ɷ��ǣ�д��ѧʽ��______��
��2����Ӧ���м���H2O2��������______��
��3�����������з�����Ӧ�����ӷ���ʽ��______��______��
��4��������IJ�����______��______�����ˡ�ϴ�ӡ����
��5���ⶨ�������崿�ȵ�ʵ�鲽�����£�
a. ȷ��ȡ3.125g����������Ʒ���100mL��Һ��
b. ȡ10.00 mL��Һ�ڴ�����ƿ�У�������ˮϡ�ͣ��������KI���壬������Ӧ��
2Cu2+ +4I��=2CuI�� + I2
c. ����������������У���μ���0.1000 mol��L-1Na2S2O3��Һ��ǡ����ȫ��Ӧ��������12. 00mL Na2S2O3��Һ��I2��2S2O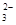 = 2I����S4O
= 2I����S4O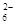
����Ʒ�е������������������д��������̣���

��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe3+ | Fe2+ | Cu2+ |
| ��ʼ���� | 1.5 | 6.4 | 4.2 |
| ��ȫ���� | 3.2 | 8.9 | 6.7 |
��2����Ӧ���м���H2O2��������______��
��3�����������з�����Ӧ�����ӷ���ʽ��______��______��
��4��������IJ�����______��______�����ˡ�ϴ�ӡ����
��5���ⶨ�������崿�ȵ�ʵ�鲽�����£�
a. ȷ��ȡ3.125g����������Ʒ���100mL��Һ��
b. ȡ10.00 mL��Һ�ڴ�����ƿ�У�������ˮϡ�ͣ��������KI���壬������Ӧ��
2Cu2+ +4I��=2CuI�� + I2
c. ����������������У���μ���0.1000 mol��L-1Na2S2O3��Һ��ǡ����ȫ��Ӧ��������12. 00mL Na2S2O3��Һ��I2��2S2O
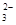 = 2I����S4O
= 2I����S4O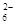
����Ʒ�е������������������д��������̣���
��1��Au��Pt ��2��ʹFe2+����ΪFe3+
��3��H+ + OH�� = H2O Fe3+ + 3OH��= Fe(OH)3��
��4������Ũ�� ��ȴ�ᾧ
��5��96%��������̲��÷֣�
�⣺n(Na2S2O3) = 0.012L��0.1000mol��L-1=" 0.0012" mol
2CuSO4��5H2O��I2��2S2O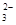
n(CuSO4��5H2O) = n(Na2S2O3) =" 0.0012" mol
m(CuSO4��5H2O) =" 0.0012" mol��250g��mol-1 = 0.30g
w(����) =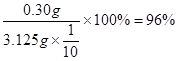
��3��H+ + OH�� = H2O Fe3+ + 3OH��= Fe(OH)3��
��4������Ũ�� ��ȴ�ᾧ
��5��96%��������̲��÷֣�
�⣺n(Na2S2O3) = 0.012L��0.1000mol��L-1=" 0.0012" mol
2CuSO4��5H2O��I2��2S2O
n(CuSO4��5H2O) = n(Na2S2O3) =" 0.0012" mol
m(CuSO4��5H2O) =" 0.0012" mol��250g��mol-1 = 0.30g
w(����) =
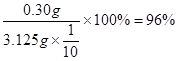
������������ڽ����������ֻ��Au��Pt���������ᣬ�����������Ҫ�ɷ���Au��Pt��
�ƹ���I����Һ�к���Cu2+��Fe3+��Fe2+��������Щ������������������ʽ����ʱ��Һ��pH��֪������H2O2��Fe2+����ΪFe3+�����ܽ���ת��ΪFe(OH)3������ȥ��
���ڳ��������м���NaOH���к�H+��H+ + OH�� = H2O�������pHʹFe3+ת��Fe(OH)3������Fe3+ + 3OH��= Fe(OH)3������
�ȹ��ˢ����Һ�к���CuSO4��ͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������塣
������ο��𰸵Ľ�����

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�
�����Ŀ